▪
Introduction
Congenital lung disease in the adult patient is rare. However, some congenital lung diseases can cause clinical symptoms for which patients undergo imaging, or these diseases may be incidentally discovered on imaging performed for other reasons. Although encountering congenital lung disease on imaging is very uncommon, the imaging appearance is often very characteristic, allowing the radiologist to make a specific diagnosis. Knowledge of the imaging and relevant clinical features is helpful for the radiologist to make the correct diagnosis and direct these patients to appropriate clinical care.
▪
Imaging Modalities
- •
Chest radiography
- •
CT of the chest and CT angiography (CTA)
- •
Thoracic MRI and MR angiography (MRA)
- •
Digital subtraction catheter angiography
- •
Imaging algorithm
Chest Radiography
In patients with congenital lung disease, the chest radiograph is frequently the initial examination performed. A typical scenario is a patient referred for chest radiography for productive cough, dyspnea, chest pain, or fever who is discovered to have a congenital lung abnormality. The clinical symptoms observed may or may not be related to the congenital lung disease. Although the chest radiograph may be suggestive for a particular congenital lung disease, cross-sectional imaging (CT or MRI) is usually required to confirm the diagnosis and to evaluate for associated abnormalities.
After the diagnosis is established, the chest radiograph may be used to monitor the stability of the lesion and evaluate for complications such as superimposed infection if clinical symptoms arise. If the patient is to undergo surgical resection, the patient may undergo chest radiography during the preoperative workup.
Chest CT and CT Angiography
CT of the chest is the most common modality used to evaluate and diagnose congenital lung disease. Due to the high spatial resolution of chest CT, it is well suited to illustrate the nature and anatomic considerations of congenital lung lesions. This includes evaluation of the anatomic location, extent of the lesion, nature of the lesion, bronchial supply, arterial supply, and venous drainage. CT angiography (CTA) is useful in cases that are vascular in nature or have important vascular anatomy. The bolus timing of the CTA should be determined by the suspected vascular supply of the lesion. For example, for lesions suspected to be of pulmonary artery origin (e.g., a pulmonary arteriovenous malformation), a bolus timing technique timed to peak enhancement of the pulmonary arteries (similar to the CT pulmonary embolism protocol) may be chosen. For lesions suspected to be of systemic arterial origin (e.g., a bronchopulmonary sequestration), a bolus timing technique timed to the descending aorta may be chosen.
Thoracic MRI and MR Angiography
MRI of the chest can also be used to evaluate congenital lung lesions, especially for younger patients in whom radiation is an important clinical concern. However, MRI is not as useful for evaluation of the lung and airways. Therefore, chest CT with IV contrast is usually chosen as the initial modality for evaluation of adult patients with suspected congenital lung disease.
However, thoracic MRI excels at evaluation of cystic lesions in the mediastinum. Therefore, thoracic MRI may be used as the primary imaging modality for the evaluation of suspected mediastinal foregut duplication cysts, such as bronchogenic or esophageal duplication cysts. Mediastinal MRI is very useful as a problem-solving technique in patients with a suspected cystic mediastinal lesion on CT but for whom the measured CT attenuation values are not diagnostic for a cyst. MRI is an excellent modality to differentiate cystic and solid lesions. MRI of the thorax performed pre- and postgadolinium, with subtraction of precontrast from the postcontrast signal, can detect even a subtle enhancing component.
MR angiography (MRA) is sometimes used to define the arterial supply of a lung or mediastinal lesion when the arterial supply is suspected to be of systemic origin. To ensure adequate spatial resolution and avoid respiratory or cardiac motion artifacts, thoracic MRA requires meticulous technique, particularly for smaller vessels. For these reasons, and for evaluation of the airways, chest CTA is usually preferred due to its higher spatial resolution, speed, and ease of imaging.
Catheter Angiography
Catheter angiography can also be used to define the arterial supply, venous drainage, and general vascularity of a congenital lung lesion. Catheter angiography is most often used as a problem-solving technique in patients in whom the vascular anatomy requires further definition prior to surgical resection. Occasionally, congenital lung lesions are embolized as a primary therapy or prior to surgical resection. In these circumstances, angiography will be performed prior to embolization.
▪
Diseases
Bronchopulmonary Sequestration
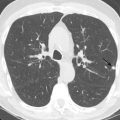
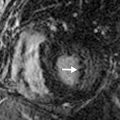
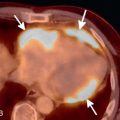
A bronchopulmonary sequestration is a mass of abnormal lung tissue that lacks normal communication with the tracheobronchial tree. The mass usually consists of abnormal, nonfunctioning lung tissue that derives its arterial supply from a systemic artery rather than a pulmonary artery. The systemic artery may be a single systemic artery or multiple arteries most commonly arising from the descending aorta, but may arise from the abdominal aorta, celiac artery, splenic artery, or rarely an intercostal artery. The identification of the systemic artery is a key feature for diagnosis of bronchopulmonary sequestration on imaging ( Fig. 15.1A–D ).








Types of Sequestration
There are two types of bronchopulmonary sequestration that are differentiated by their pleural covering and venous drainage. Intralobar sequestration is more common, comprising 75% of sequestrations, and the sequestered lung tissue is located within the visceral pleura of the affected lobe. Therefore an intralobar sequestration may communicate through collateral air drift with the normally aerated lung of the affected lobe. In extralobar sequestration, the sequestered lung tissue is enclosed within its own pleural envelope. This unique pleural covering precludes communication with other parts of the lung. The presence of air within the sequestration indicates that the lesion is most likely an intralobar sequestration because intralobar sequestrations can communicate with the lung through collateral air drift, whereas the extralobar sequestration is enclosed and should not contain air.
The venous drainage of intralobar and extralobar sequestration is sometimes a differentiating feature but is not universally consistent. The venous drainage of intralobar sequestrations tends to be to pulmonary veins, whereas the venous drainage of extralobar sequestrations tends to be to systemic veins, such as the azygous vein, hemiazygos vein, or inferior vena cava (IVC).
Imaging Appearance
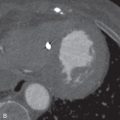
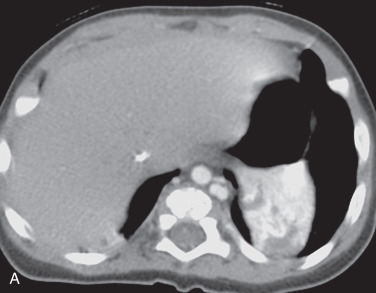
Intralobar sequestrations may present as a solid mass or cystic mass. Cystic intralobar sequestrations may contain air cysts, fluid cysts, or both air- and fluid-filled cysts and there may be air-fluid levels (see Fig. 15.1E–H ). Rarely, an intralobar sequestration may present as a focal area of hyperinflated lung. Extralobar sequestration typically presents as a solid vascular mass in the lung base, near the hemidiaphragm ( Fig. 15.2 ). In all cases of sequestration, a systemic artery is present, and normal bronchial communication is absent.



Complications
The most common complication of intralobar bronchopulmonary sequestration is superinfection. This complication is unique to intralobar sequestration due to the potential collateral air drift or fistulous communication of the sequestration with other parts of the lung. Extralobar sequestrations rarely become infected because they are completely enclosed. Deciding whether an infection is present may be difficult on imaging. However, if there is prior imaging, any new consolidation or air-fluid levels may suggest superimposed infection.
Other rare complications of intralobar sequestrations may include hemoptysis, hemothorax, and high-output cardiac failure from left-to-right or left-to-left shunting. Rare complications of extralobar sequestration include hemothorax, infarction, torsion, and high-output failure from shunting.
Goals of Imaging
The goals of imaging are to identify the feeding artery and its origin because identification of the artery is essential to the diagnosis and potential management. It is also important to identify if there is more than one feeding artery for a surgeon who may be planning a surgical resection.
Identify the venous drainage if possible. This may suggest whether the lesion is intralobar or extralobar and is also important information for a surgeon.
Delineate the extent of the lesion. Identify the full anatomic extent of the lesion, the lobe involved, and whether there is any involvement below the diaphragm. Extralobar sequestrations have a higher incidence of an ectopic location.
Describe any features that may suggest a complication, such as superimposed infection.
Differential Diagnosis
- 1.
It is important to distinguish sequestration from a malignancy such as a primary lung malignancy or metastasis. These would usually be differentiated by identification of the systemic arterial supply. Extremely rarely, a malignancy may occur in conjunction with a sequestration and may be suggested by an increasing or enhancing mass within the lesion.
- 2.
Systemic arterial supply to normal lung may arise from hypertrophy in chronic inflammation, chronic pulmonary embolism, or the absence of an interlobar or segmental pulmonary artery. The diagnosis of a sequestration requires the concurrent absence of normal bronchial communication to the affected lung parenchyma in addition to the systemic arterial supply.
- 3.
Certain congenital lung diseases may have a systemic arterial supply to portions of the lung in the absence of bronchopulmonary sequestration. These diseases have normal bronchial communication to the affected lung.
Treatment
Bronchopulmonary sequestration is usually excised by open thoracotomy or video-assisted thoracic surgery. This may be a segmentectomy or lobectomy, depending on the anatomy of the lesion. Transcatheter embolization has been used in select cases, with variable success.
Congenital Pulmonary Airway Malformation
Congenital pulmonary airway malformations (CPAMs) comprise a spectrum of airway malformations (hamartomas) that involve various portions of the tracheobronchial tree. CPAMs were previously referred to as congenital cystic adenomatoid malformations (CCAMs), but the name of the disease has been changed because not all these lesions are adenomatoid or cystic. The classification scheme for CPAMs has also been expanded, and there are now five types of CPAM based on the pathologic site of origin of the lesion in the tracheobronchial tree:
Type 0: Airway malformations of the proximal tracheobronchial tree
Type 1: Airway malformations of bronchial or bronchiolar origin (large cyst lesion)
Type 2: Airway malformations of bronchiolar origin (the small cyst lesion)
Type 3: Airway malformations of bronchiolar or alveolar duct origin (the adenomatoid lesion)
Type 4: Airway malformations of distal acinar or alveolar origin (the unlined cyst lesion)
Imaging Appearance
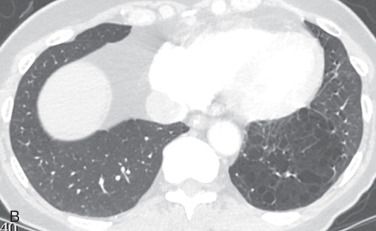
Usually CPAMs present early in life but have been reported in adult patients. At imaging, CPAMs usually present as a cystic mass ( Fig. 15.3 ), but occasionally appear solid. If the largest cyst is >2.5 cm, these tend to be type 1 CPAMs. If the cysts are <2.5 cm or the lesion is solid, it is difficult to predict the subtype of CPAM. The diagnosis of a CPAM in an adult patient is controversial due to overlap of the imaging features of CPAM and lung injury or repair, which may result in cystic lesions or pneumatoceles. Usually, CPAMs have normal arterial and venous connections, differentiating CPAM from bronchopulmonary sequestration.



Complications
The classic presentation of CPAM is respiratory distress in a newborn patient. In older patients, CPAM may present as recurrent pneumonia in the region of a multicystic lesion on CT. Less commonly, patients may present with spontaneous pneumothorax, and there is a rare association with adenocarcinoma (especially for type 1 CPAM).
Differential Diagnosis
The following are part of the differential diagnosis for a CPAM:
- 1.
Postinflammatory cystic lesion. This may be difficult to differentiate from CPAM, making the diagnosis of CPAM in adult patients controversial.
- 2.
Bronchopulmonary sequestration. This would be distinguished by the presence or absence of a systemic artery supplying the lesion.
- 3.
Cystic malignancy, such as adenocarcinoma or cystic metastasis. The cysts associated with CPAM typically have thin walls. Thickening or irregularity of the wall of a cyst should raise concern for a cystic malignancy or a superimposed malignancy.
- 4.
Bullous emphysema, cystic lung disease, or emphysema. This is usually diffuse and involves both lungs.
Congenital Lobar Overinflation
Congenital lobar overinflation (CLO) is the result of the congenital partial obstruction of a bronchus, with progressive overinflation of the affected lobe. It has been postulated that there is a ball valve obstruction with overinflation during inspiration and distal air trapping in expiration. The result is progressive overinflation of the affected lobe. In about 50% of cases, the cause is unknown, but known causes include mucosal flaps, deficient cartilage, airway stenosis or malacia, and an extrinsic mass (bronchogenic cyst, a vessel such as patent ductus arteriosis, or vascular ring). The classic presentation is respiratory distress in infancy, and only 5% of cases present after the age of 6 months.
Imaging Appearance
On chest radiography, CLO presents as overinflation of a lobe, usually the left upper lobe (41%), right middle lobe (34%), or right upper lobe (21%). There is displacement of the mediastinum away from the affected lobe, depression of the diaphragm, and compressive atelectasis in the adjacent lobe ( Fig. 15.4A ). In the postnatal period, the lobe may be filled with amniotic fluid, which clears in days.


On chest CT, there is severe lobar overinflation. There will be increased volume within the affected lobe, hyperlucency, attenuation of pulmonary vessels, and a contralateral mediastinal shift (see Fig. 15.4B ). Atelectasis may also be seen in the unaffected lobe. CT confirms lobar overinflation and excludes the radiographic differential considerations of hypoplasia, proximal interruption of the pulmonary artery, or scimitar syndrome. CT may also reveal an underlying cause.
Foregut Duplication Cysts: Bronchogenic and Esophageal Duplication Cysts
A bronchogenic cyst is a congenital lesion thought to be secondary to abnormal budding of the embryonic foregut. The separated focus of tracheal-bronchial tissue fails to develop further, resulting in a cyst. Two-thirds of bronchogenic cysts occur in the mediastinum and one-third occur in the lung. Connection with a bronchus is unusual, but may occur if the lesion becomes infected or is instrumented.
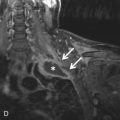
Esophageal duplication cysts occur due to abnormal esophageal development and recanalization. Due to the elongation and rotation of the foregut, esophageal duplication cysts are usually found along the lower esophagus and on the right ( Fig. 15.5 ).

Imaging Appearance
On chest radiography, a mediastinal foregut duplication cyst (FDC) may appear as a mediastinal mass or abnormal mediastinal interface, usually in the azygoesophageal recess or right paratracheal region ( Fig. 15.6A ). An intrapulmonary bronchogenic cyst may appear as a spherical mass, most often medially in the lower lobes.


Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree