Congenital cerebral vascular anomalies include a spectrum of conditions that result from perturbation of normal developmental processes. Although some of these conditions are asymptomatic and well compensated by collateral circulation, others can cause significant morbidity or produce a range of complications for affected patients. Knowledge of the underlying developmental etiologies and the associated imaging characteristics helps fully elucidate the morphologic and hemodynamic details of these lesions and determine the necessity for any intervention.
Congenital arterial and venous anomalies of the brain and skull base comprise diverse abnormalities that result from early disturbances in cerebrovascular development. Broadly, these conditions may result in one or more of the following structural abnormalities: absence or hypoplasia of normal vessels, persistence of transient embryonic vessels, vessels with abnormal morphology, and pathologic intracerebral shunts (arteriovenous malformations [AVM] and arteriovenous fistulae [AVF]). Some of these anomalies are hemodynamically compensated and lack clinically significant symptoms. These abnormalities often go unrecognized or are only detected incidentally on imaging studies. Others can cause significant morbidity and mortality from hemorrhagic or ischemic sequelae and warrant appropriate identification and management. Knowledge of the underlying etiology and associated imaging findings is essential in properly differentiating lesions with benign nature from those that have significant clinical implications and may need management. Full description of complex embryologic processes is beyond the scope of this article and the reader is referred to the works of Streeter and Padget. The authors’ aim is to provide a brief overview of normal cerebrovascular development and embryologic pathogenesis of common, congenital arterial and venous anomalies of the cerebral vasculature and highlight key imaging characteristics that should be considered for their diagnosis.
Overview of normal cerebrovascular development
Vascular development of the brain is driven at the molecular level by angiogenic signaling cascades and at the morphologic level by the metabolic demands of the growing central nervous system. At 2 to 4 weeks of gestation, the neural plate is still primarily fed by free diffusion of nutrients from the amniotic fluid. By 4 weeks, the neural tube has closed and the neuroectoderm begins to derive nutrients from the surrounding meninx primitiva, a mesodermal structure that will give rise to the future calvarium, dura, and leptomeninges. Although primordial cerebral vessels can be seen within the meninx primitiva at this early stage, the channels are largely arranged in an indistinct network and have limited arterial or venous differentiation. The mature framework of the proximal cerebral arterial system is laid out by 8 weeks of gestation and that of the venous system is laid out by 12 weeks. Distal vessel development and remodeling continue until birth for the arterial system and continues postnatally for the venous system. Details of arterial and venous morphogenesis and their implications in the development of anomalous vessel anatomy are discussed in subsequent sections.
Arterial system
Internal Carotid Arteries
Development
The internal carotid arteries (ICA) play an essential role in supplying the vesicles of the developing brain and appear early in gestation, before closure of the neural tube. These paired structures can first be seen arising from the dorsal aorta and third aortic arch at the 3- to 5-mm embryonic stage. By the 4- to 5-mm (28–29 day) stage, the primitive ICA lies within the vascular meshwork of the meninx primitiva and provides blood supply to the forebrain, midbrain, and hindbrain vesicles. A more mature ICA can be seen by 6 weeks, although its branches continue to develop beyond that point.
The widely accepted system proposed by Lasjuanias and Santoyo-Vazquez, divides the ICA into 7 segments, each bordered by an embryonic precursor vessel. The cervical segment (first) of the ICA starts at the common carotid artery bifurcation and ends at its entrance into the skull base. The ascending petrous segment (second) begins as the ICA enters the skull base through the carotid foramen and extends to the apex of the anterior-medial curvature within the petrous bone, marked by the caroticotympanic artery distally. The horizontal petrous segment (third) traverses the carotid canal from the caroticotympanic artery to the vidian artery where the ICA emerges from the skull base through the foramen lacerum. The ascending cavernous segment (fourth) enters the cavernous sinus (CS) and ends at the meningohypophyseal trunk (MHT) where the ICA assumes an anterior-inferior course within the CS. The MHT is likely an adult derivative of the primitive trigeminal artery (TA), whereas the inferior hypophyseal artery, which branches from the ICA medial and distal to the MHT, is speculated to be a derivative of the medial branch of the primitive maxillary artery. The horizontal cavernous segment (fifth) extends from the MHT to the inferolateral trunk (ILT) within the CS. The clinoidal segment of the ICA (sixth) is bordered proximally by the ILT and distally by the adult ophthalmic artery (OA). The terminal segment (seventh) of the ICA lies between the primitive OA and anterior cerebral artery.
Carotid artery agenesis and hypoplasia
Congenital absence of the ICA is a rare developmental abnormality with incidence reported at approximately 0.01% and is often discovered incidentally on head and neck imaging. ICA hypoplasia is more common, with an incidence of 0.079%. Improper development of one or more of the 7 different embryologic segment branches that form the ICA can lead to poor formation of the carotid system.
Unilateral absence of the ICA is generally asymptomatic, because sufficient collateral pathways usually exist via the circle of Willis, intracavernous anastomoses, and persistent embryologic arteries. Occasionally, in the presence of insufficient collaterals, symptoms related to cerebral ischemia from uncompensated blood flow can occur. Absence of one ICA can result in excessive shear stress from increased flow in contralateral ICA or other associated collaterals. These hemodynamic changes can lead to the development of intracranial aneurysms. Congenital absence of an ICA can sometimes be confused with acquired occlusions. Similarly, congenital hypoplasia can be mistaken as acquired stenosis. It is important to understand the presentation, imaging features, and clinical implications of these entities because improper diagnosis caused by associated symptoms may lead to unnecessary and risky interventions.
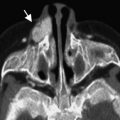
The typical morphology of a hypoplastic ICA is an artery of small caliber that becomes diminutive at, or shortly after, its origin. Commencement of the hypoplasia 1 to 2 cm past the bifurcation is a common finding. It remains small throughout its course and may either continue intracranially or become completely occluded. The key-imaging finding is the absence of petrous bony carotid canal in ICA agenesis and smaller canal size in ICA hypoplasia ( Fig. 1 ). The bony carotid canals normally develop at the fifth and sixth embryonic weeks following the completion of ICA development at the fourth week. ICA development serves as a stimulus without which carotid canals do not develop. Demonstration of a small or absent carotid canal by computed tomography (CT) helps to differentiate from acquired stenosis or occlusion.
Aberrant internal carotid artery
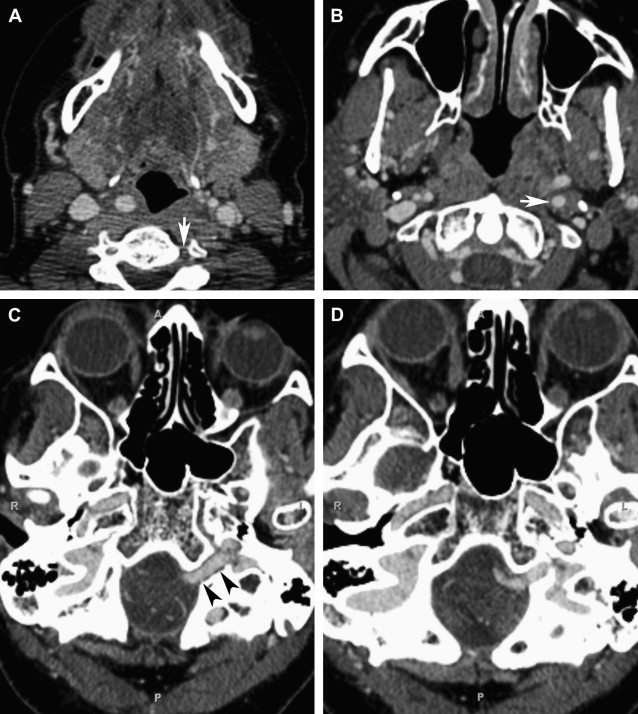
The ICA normally ascends in the neck and enters into the temporal bone via the carotid canal. As it courses through the carotid canal, a thin plate of bone, the carotid ridge, separates it from the posteriorly located internal jugular vein. The artery then runs superiorly to form the initial vertical segment, anterior to the cochlea, and is separated from the tympanic cavity by a thin plate of bone. The ICA then turns anteriorly (inferoposterior and medial to the eustachian tube), traverses the foramen lacerum, and enters the middle cranial fossa. An aberrant ICA, on the other hand, enters the floor of the middle ear, turns forward, and then passes through the middle-ear instead of staying anterior to this space before entering the foramen lacerum. Although termed aberrant ICA, the basic pathology in this condition is thought to be hypoplasia or agenesis of the ICA. In the setting of ICA agenesis, the inferior tympanic artery does not undergo normal regression and instead hypertrophies. It is this hypertrophied inferior tympanic artery that appears as aberrant ICA and anastomoses with the caroticotympanic artery to carry the blood to the carotid siphon.
An aberrant ICA can present at any age and is more commonly seen in women, on right side. Clinical diagnosis of aberrant ICA can be difficult because of the lack of any pathognomonic symptoms. Patients may be completely asymptomatic or can present with nonspecific signs and symptoms, such as conductive hearing loss, pulsatile tinnitus, ear fullness, otalgia, and vertigo. Pulsatile tinnitus and hearing loss are caused by contact between the malleus handle and the exposed carotid artery. The pulsatile bruit can often be heard with a stethoscope in the ear canal or around the ear. The presentation of an asymptomatic middle-ear mass is common. It can also present as a vascular mass behind the tympanic membrane on otoscopic examination. An aberrant ICA can be confused with a glomus tumor, dehiscent jugular bulb, cholesterol granuloma, or petrous carotid aneurysm.
It is important to recognize aberrant ICAs because misdiagnosis of this entity may subject the patients to potentially life-threatening iatrogenic ear bleeding. There are several reported cases when aberrant ICA was first recognized during middle-ear surgery or myringotomy. Hemorrhage can occur from surgical manipulation, biopsy, or myringotomy. If an aberrant ICA is suspected within the middle ear on imaging, it is crucial to inform the clinician to avoid any inappropriate surgical intervention.
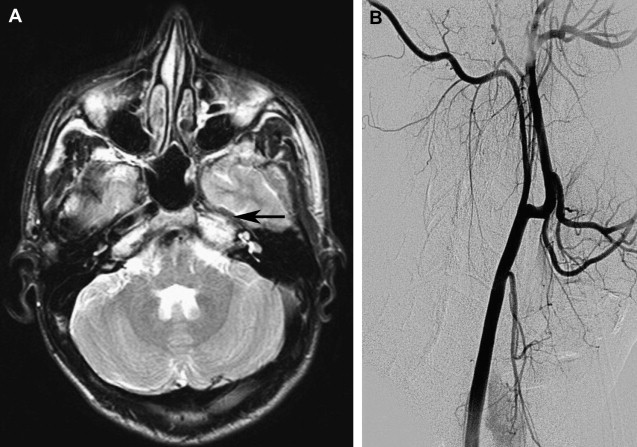
An aberrant carotid artery can be evaluated with several available radiologic techniques, including CT, CT angiography (CTA), magnetic resonance angiography (MRA), or digital subtraction angiography (DSA). CT with high-resolution imaging is the study of choice because of its capability to provide exquisite bony details. The aberrant carotid artery can easily be seen entering the middle ear through an enlarged inferior tympanic canal that lies posterior to the normal carotid canal. This entrance point of ICA into the tympanic canal can also be seen as a focal narrowing ( Fig. 2 ) on angiography.
Intracranial Arterial Vasculature: Embryology and Variations
The arterial vasculature of the brain can be described in the context of anterior circulation, supplied primarily by the ICAs, along with contributions from the external carotid artery (ECA) and posterior circulation, supplied by the vertebral arteries (VA). The development of the cerebral arterial system mirrors the corresponding metabolic demands of the growing cephalic vesicles. The formation of the circle of Willis occurs progressively with staggered development of the anterior and posterior circulation. The primitive anterior circulation can be well recognized by 35 days of gestation, with anterior cerebral artery (ACA) and middle cerebral artery (MCA) branches from the rostral ICA. The primitive posterior circulation is completed later in development (6–7 weeks), with capture of the hindbrain vascular territory by the vertebrobasilar system. Although the posterior communicating artery (PCOM) forms at the 29-day stage, the posterior circulation continues to be maintained by the proatlantal artery (ProA) until 6 weeks of gestation. The circle of Willis is finalized by 6 to 7 weeks of gestation, with formation of the posterior cerebral arteries and cerebellar arteries.
Distal vessels are generally less variable in morphology because the final vascular architecture is restricted by the parenchymal metabolic requirements, but the proximal vessels (ie, at the circle of Willis) can vary far more, depending on early hemodynamic factors. A complete discussion of congenital variations of the circle of Willis is outside the scope of this article, but it is important to mention that anatomic variations here are the rule rather than the exception. Remodeling continues throughout life depending on individual hemodynamic factors, so the ultimate adult morphology can be further altered from the immediate postnatal appearance.
Many of the cerebral vessels develop from initial plexiform networks and rely on proper fusion of branching segments to form a single adult channel. Interruptions to this process can cause fenestrations, which are characterized by division of the artery lumen into 2 distinct, parallel, endothelium-lined channels. Specific locations and etiologies of these anomalies are subsequently discussed for each cerebral artery. The overall angiographic incidence of fenestrations ranges from 0.03% to 1.0%, and the postmortem incidence is 1.3% to 5.3%, suggesting that many of these lesions can be missed on imaging studies. These lesions are generally asymptomatic, but similar to arterial bifurcations, they may have a propensity for aneurysm formation. On conventional DSA, fenestrations can be obscured depending on the viewing angle and because of overlapping structures. However, modern 3-dimensional rotational angiography and CT/MR angiography with multiplanar reconstructions and rotational capabilities have allowed for better detection of these abnormalities.
Anterior cerebral artery
The ACA is the first cerebral artery visible in development. It emerges from a vascular meshwork as a distinct trunk by 32 days and extends rostrally around the growing hemispheric vesicle. At 35 days, the paired ACAs approach the midline to form the anterior communicating artery (ACOM), which is plexiform at this stage and continues to remodel until full development of the cerebrovascular system. Improper fusion of these branches into a single channel can result in arterial fenestration. The ACOM is among the more common locations for fenestrations in the cerebral circulation. At day 40, important modifications to the A2 and distal ACA segments produce the classic paired ACA anatomy; insults during this period can result in anomalous morphologies, such as bihemispheric or azygous ACAs.
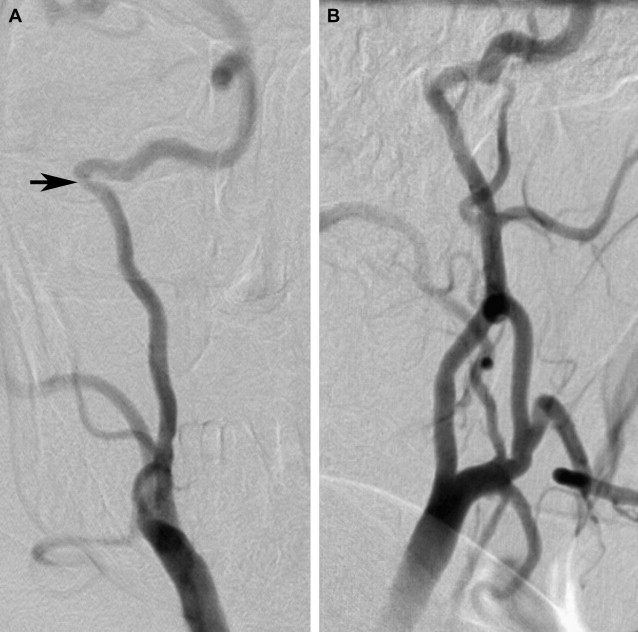
A bihemispheric ACA describes the presence of a dominant A1 segment that gives supply to the distal paired ACA segments for both hemispheres. The contralateral A1 segment is typically hypoplastic or has an early termination. Bihemispheric ACAs are more common than azygous ACAs in which the paired ACA pattern is replaced by a fused midline vessel. The azygous or undivided ACA is a single, midline vessel arising from the confluence of the two A1 segments. It supplies the medial aspects of the frontal and parietal lobes of both hemispheres. Compression of the contralateral carotid artery during DSA can help differentiate the presence of an azygous ACA from the more common bihemispheric ACA. Azygous ACAs can be well demonstrated on CTA, MRA, and DSA. An azygous ACA can be associated with midline central nervous system malformations, such as agenesis of corpus callosum, holoprosencephaly, intracranial arteriovenous malformation, and aneurysm. Both saccular and nonsaccular aneurysms have been described in relation to azygous ACA ( Fig. 3 ). A complex branching pattern of the distal azygous ACA often contributes to the unusual morphology of these aneurysms. Such aneurysms are often challenging for both clipping and endovascular repair because of their nonsaccular morphology and difficult visualization of the efferent arterial branches.
Middle cerebral artery
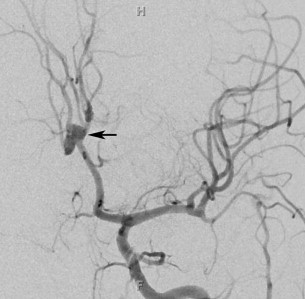
The MCA forms as a basal striatal branch of the primitive ACA starting at 35 days of development, although its stem can be visible as early as 32 days. It typically forms a single channel from the coalescence of 2 or 3 initial vessel stems. Full maturation of the MCA continues until 7 to 8 weeks, when the proximal framework of the cerebral arterial system is fully established. Various mechanisms have been proposed for development of different fenestrations. Early branching of the temporopolar artery could possibly interrupt the normal coalescence of the primordial MCA branches and can lead to the appearance of fenestrations in postnatal artery ( Fig. 4 ).
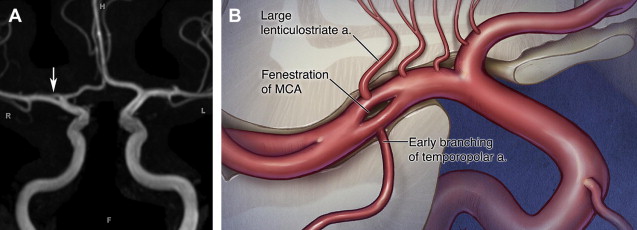
MCA duplication refers to the early origin of the MCA from the distal ICA. This anomaly can be observed on angiographic and anatomic studies at a frequency of 0.2% to 2.9%. The duplicated MCA is typically a small vessel that supplies the anterior temporal lobes and has little clinical significance.
An accessory MCA refers to the origin of the MCA from the proximal or distal A1 segment. The frequency of this anomaly has been reported between 0.3% and 4.0% on angiographic and anatomic studies. Because the MCA is a phylogenetic branch of the ACA, investigators have suggested that the accessory MCA is a vestigial remnant of this embryonic anastomosis. Others have proposed that this morphology is in fact a medial extension of the artery of Heubner into the territory of the MCA. Increased hemodynamic turbulence at the accessory MCA origin has been also proposed to be associated with increased risk of aneurysm formation.
Posterior cerebral and anterior choroidal arteries
The development of the posterior cerebral artery (PCA) in the fetal brain occurs late and arises from the fusion of several embryonic vessels near the caudal end of the PCOM. The PCA begins as a continuation of the PCOM in the majority of cases, with the remainder of the PCAs originating from the basilar artery (BA). In adults, it has the most variable morphology of all the cerebral arteries. It can be supplied by the ICA with the prominent P1 segment and persistent embryonic anastomoses or relegate its territory to the anterior choroidal artery (AchA). Depending on the relative contribution, the PCA can be classified as adult type when the P1 segment of the PCA is larger than the ipsilateral PCOM artery, transitional type when the P1 segment is of the same size as the PCOM artery, and fetal type when the P1 segment is smaller (or absent) than the PCOM artery. The anterior choroidal artery is one of the most prominent arteries in the developing brain. At 32 days of gestation, it is the largest branch of the ICA and supplies much of the primitive diencephalon. It maintains this region until 7 to 8 weeks, at which point the growing posterior cerebral artery overtakes much of this territory. By birth, the territory of the AchA is normally limited to only small regions of its original embryonic territory.
In rare cases, the postnatal anterior choroidal artery can become hypertrophic and supply the entire hemispheric territory that is typically vascularized by the posterior cerebral artery. Embryologically, this is most likely caused by errors at the 7- to 8-week period when the developing PCA should assume dominance in these regions.
Vertebrobasilar arterial system
The developing hindbrain is initially fed by paired longitudinal neural arteries (LNAs) and transient anastomoses from the ICAs. At 29 gestational days, the LNAs begin as a craniocaudal, midline fusion to form the primitive BA, and at 32 gestational days, the vertebral arteries begin to form from longitudinal anastomoses between cervical intersegmental arteries. Although the vertebrobasilar framework is established at this point, it is not until 7 to 8 weeks that the rhombencephalic and mesencephalic vascular territories of the PCA are taken over by the vertebrobasilar system from the ICAs.
Fusion of the basilar artery occurs in longitudinal and axial planes, and failure of these normal processes on either axis can lead to abnormal vessel morphologies. Longitudinal fusion typically begins in a disparate fashion, with segmented unfused regions that only secondarily become a unified channel. Failure of this secondary fusion can leave residual fenestrations in the vessel, which is seen most commonly in the caudal BA. Axial nonfusion occurs by a similar mechanism and results in discontinuities within the BA. This discontinuity can result in a segmented vertebrobasilar architecture that is fed by collateral paths from other anomalous vessels, like a persistent trigeminal artery, discussed later. If a fenestration is visualized, careful attention should be given to look for any associated aneurysm.
Persistent Carotid-Basilar Anastomoses
By the time the human embryo is 4 to 5 mm long, the groundwork of anterior circulation is established as the primitive ICAs reach the developing forebrain. The paired LNA, precursors of the BA, also form on the medial edges of the bilateral vascular networks on the ventral surface of the hindbrain. The first few small branches to be sent out from the ICA are responsible for delivering blood to the LNA to feed the developing hindbrain while the vertebrobasilar system is under construction. These branches include 3 transient presegmental arteries (carotid-basilar anastomoses), 1 permanent presegmental artery, and the first intersegmental artery. These arteries also likely contribute to the formation of the LNA by anastomosis.
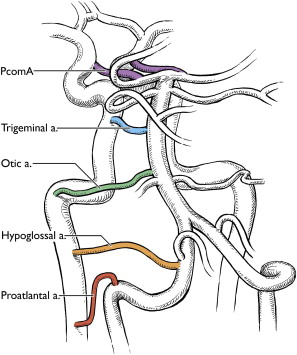
The 3 presegmental arteries include the TA, the otic artery (OA), and the hypoglossal artery (HA), named for their associations with the fifth, eighth, and twelfth cranial nerves, respectively. All 3 of these arteries are present in embryos of 4 to 5 mm in length. The trigeminal artery can be observed, at a 3-mm embryo length, branching from the ICA to the LNA directly opposite to the first arch at the level of the trigeminal ganglion. This branch remains the chief supply to the developing LNAs at a 4-mm embryo length, augmented caudally by the OA, the HA, and the ProA.
The OA has been reported in 4-mm embryos opposite the second arch travelling with the vestibulocochlear nerve and the otic vesicle. It regresses concurrently with the second branchial arch artery. Although definitive case reports of persistent TAs, HAs, and first intersegmental arteries can be found within the medical literature, there are only a few reported cases of a persistent OA with angiographic documentation. These cases better meet the criteria of a low-originating persistent primitive trigeminal artery, and true existence of the OA is under debate.
The HA originates from the ICA, following the course of the developing hypoglossal nerve and connecting to the LNA. It becomes visible at roughly a 4-mm embryo size as the second arch starts to regress, and remains patent for only a short period of time, dwindling as the LNA start to fuse into the BA.
By a 5- to 7-mm embryo length, the primitive posterior communicating artery (PcomA) forms a permanent connection from the ICA to the LNAs, and TAs consequently begin to diminish in caliber. Few investigators have proposed that the PcomA most likely represents the cranial-most presegmental artery and refer to the PcomA as the first presegmental artery in addition to the 3 previously described presegmental arteries. Of these presegmental arteries, only the PcomA normally persists into adulthood, whereas the remaining 3 regress early on in development.
The involution of the trigeminal artery is also dependent on the establishment of the first intersegmental artery, termed the proatlantal artery. By the time the embryo is 4 to 5 mm in length, the ProA is the primary caudal source of blood for LNAs. The paired LNAs begin to merge around the time that the embryo is 5 mm. Rather than shifting medially, the LNAs coalesce along the midline of the developing hindbrain as they increase in diameter. The fused LNA of a 12- to 14-mm embryo now represent the freshly formed BA.
Bilateral channels form between the cervical segmental arteries that connect the developing subclavian arteries to the ProA once the embryo is 7 to 12 mm. It is not until this longitudinal paravertebral anastomosis, representing the future V1 segment of the VA, is complete that the proximal segment of the ProA regresses and its remaining portion is incorporated into the VA. Like every intersegmental artery, the ProA provides a dorsospinal division with a radicular artery. The radicular artery of the ProA becomes the final segment of the adult VA. The anterior and posterior radicular branches of the ProA divide just before crossing the dura so that the anterior radicular artery serves as the V4 segment of the VA and the posterior radicular artery forms the posterior spinal artery.
The carotid-basilar anastomoses normally disappear during fetal development, but may persist in adult life with overall incidence of 0.1% to 1.0%. Among all persistent carotid-basilar anastomosis ( Fig. 5 ), the persistent trigeminal artery (PTA) is the most common and is the most cephalic in location. PTA arises from the posterior genu of the cavernous segment of ICA and joins the cephalic end of the BA adjacent to the clivus. It can either have a parasellar course when it passes lateral to the dorsum sellae or an intrasellar course when it passes medially and actually perforates the dorsum sellae. It can predominantly be seen in 3 types of patterns. In the Saltzman type I, it joins the BA between the superior cerebellar arteries and anterior inferior cerebellar arteries. The BA proximal to the junction is usually hypoplastic and the posterior communicating arteries are absent or poorly opacified. PTA supplies the entire vertebrobasilar system distal to the anastomosis. In Saltzman type II, it also joins the BA between the superior cerebellar arteries and the anterior inferior cerebellar arteries, but the posterior communicating arteries are present and supply the posterior cerebral arteries. The Saltzman type III refers to the trigeminal artery variant when it directly joins to the cerebellar artery.
As previously discussed, the persistent OA has been rarely reported with only a handful of cases published and many question its very existence. To be labeled as OA, it must originate from the lateral-most portion of the petrous segment of the ICA and then traverse through the internal auditory meatus before joining the caudal end of the BA. None of the reported cases have shown this detail in a convincing manner.
The persistent hypoglossal artery (PHA) is the second most common after the PTA. This vestigial vessel normally regresses during week 5 of embryogenesis. The persistent vessel comes off ICA at the C1 to C3 level, enters the skull through the anterior condylar or hypoglossal canal and courses posteromedial to continue as the terminal segment of the vertebral artery and the BA ( Fig. 6 ). The contralateral vertebral artery, if present, generally terminates in the posterior inferior cerebellar artery (PICA).
The primitive proatlantal intersegmental artery generally disappears during week 6, at the time when the vertebral artery becomes functional. The persistent artery is the proximal segment of this artery, whereby the vertebrobasilar circulation derives supply either from the internal or external carotid arteries. A persistent proatlantal intersegmental artery type I usually originates from the extracranial ICA to join the vertebral artery between the occipital bone and C1. The type I vessel does not traverse a foramen transversarium. A type II persistent proatlantal intersegmental artery, on the other hand, originates from the external carotid artery and joins the normal course of the vertebral artery at the C1-C2 interspace.
The clinical significance of various persistent carotid-basilar anastomoses lies in understanding and recognizing associated anatomic variations and vascular disorders. These persistent vestigial arteries are often noticed incidentally and probably have a low incidence of direct clinical impact. The reports of various clinical presentations are likely biased by the fact that the suggested associated disorders often lead to vascular investigations. Nevertheless, PTA has been associated with various vascular anomalies in up to 25% of the cases. These anomalies include carotid-cavernous fistula ( Fig. 7 ), aneurysms, Sturge-Weber syndrome, hemangioma of the head and neck, cerebral AVM, and other arterial anomalies. Other persistent anastomoses have been shown to be associated with similar anomalies.
It is important to understand these anatomic variations along with associated vascular disorders because they warrant appropriate modifications of interventional neuroradiology procedures. Recognizing the trigeminal artery is critical during the Wada test to avoid infusion of barbiturates into the posterior fossa. While treating lesions associated with a trigeminal artery and mid-basilar atresia or hypoplasia, it is vitally important to preserve the trigeminal artery, which is the main source of flow to the posterior circulation. Similarly, appropriate modifications are important to safely perform endovascular balloon occlusion tests and open surgical procedures when such lesions are present.
Arterial system
Internal Carotid Arteries
Development
The internal carotid arteries (ICA) play an essential role in supplying the vesicles of the developing brain and appear early in gestation, before closure of the neural tube. These paired structures can first be seen arising from the dorsal aorta and third aortic arch at the 3- to 5-mm embryonic stage. By the 4- to 5-mm (28–29 day) stage, the primitive ICA lies within the vascular meshwork of the meninx primitiva and provides blood supply to the forebrain, midbrain, and hindbrain vesicles. A more mature ICA can be seen by 6 weeks, although its branches continue to develop beyond that point.
The widely accepted system proposed by Lasjuanias and Santoyo-Vazquez, divides the ICA into 7 segments, each bordered by an embryonic precursor vessel. The cervical segment (first) of the ICA starts at the common carotid artery bifurcation and ends at its entrance into the skull base. The ascending petrous segment (second) begins as the ICA enters the skull base through the carotid foramen and extends to the apex of the anterior-medial curvature within the petrous bone, marked by the caroticotympanic artery distally. The horizontal petrous segment (third) traverses the carotid canal from the caroticotympanic artery to the vidian artery where the ICA emerges from the skull base through the foramen lacerum. The ascending cavernous segment (fourth) enters the cavernous sinus (CS) and ends at the meningohypophyseal trunk (MHT) where the ICA assumes an anterior-inferior course within the CS. The MHT is likely an adult derivative of the primitive trigeminal artery (TA), whereas the inferior hypophyseal artery, which branches from the ICA medial and distal to the MHT, is speculated to be a derivative of the medial branch of the primitive maxillary artery. The horizontal cavernous segment (fifth) extends from the MHT to the inferolateral trunk (ILT) within the CS. The clinoidal segment of the ICA (sixth) is bordered proximally by the ILT and distally by the adult ophthalmic artery (OA). The terminal segment (seventh) of the ICA lies between the primitive OA and anterior cerebral artery.
Carotid artery agenesis and hypoplasia
Congenital absence of the ICA is a rare developmental abnormality with incidence reported at approximately 0.01% and is often discovered incidentally on head and neck imaging. ICA hypoplasia is more common, with an incidence of 0.079%. Improper development of one or more of the 7 different embryologic segment branches that form the ICA can lead to poor formation of the carotid system.
Unilateral absence of the ICA is generally asymptomatic, because sufficient collateral pathways usually exist via the circle of Willis, intracavernous anastomoses, and persistent embryologic arteries. Occasionally, in the presence of insufficient collaterals, symptoms related to cerebral ischemia from uncompensated blood flow can occur. Absence of one ICA can result in excessive shear stress from increased flow in contralateral ICA or other associated collaterals. These hemodynamic changes can lead to the development of intracranial aneurysms. Congenital absence of an ICA can sometimes be confused with acquired occlusions. Similarly, congenital hypoplasia can be mistaken as acquired stenosis. It is important to understand the presentation, imaging features, and clinical implications of these entities because improper diagnosis caused by associated symptoms may lead to unnecessary and risky interventions.
The typical morphology of a hypoplastic ICA is an artery of small caliber that becomes diminutive at, or shortly after, its origin. Commencement of the hypoplasia 1 to 2 cm past the bifurcation is a common finding. It remains small throughout its course and may either continue intracranially or become completely occluded. The key-imaging finding is the absence of petrous bony carotid canal in ICA agenesis and smaller canal size in ICA hypoplasia ( Fig. 1 ). The bony carotid canals normally develop at the fifth and sixth embryonic weeks following the completion of ICA development at the fourth week. ICA development serves as a stimulus without which carotid canals do not develop. Demonstration of a small or absent carotid canal by computed tomography (CT) helps to differentiate from acquired stenosis or occlusion.

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree