CHAPTER 28 Congenital Cardiac Surgery
Pediatric cardiology as a specific discipline can track its beginnings to the first ligation of a patent ductus arteriosus by Gross in 1938.1 Much anatomic research had been done up to that time, but surgical treatment was now an option (Table 28-1). In 1945, Crafoord and Nylin2 reported the first surgical repair of coarctation of the aorta, and in the same year, surgical palliation of tetralogy of Fallot with an aortopulmonary shunt was described by Taussig and Blalock.
TABLE 28-1 Chronology of Selected Milestones in Pediatric Cardiology
| 1936 | Maude Abbott publishes landmark atlas with historical data on patients with congenital heart disease. |
| 1939 | Gross and Hubbard publish case reports of a 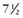 -year-old patient with successful ligation of a patent ductus. -year-old patient with successful ligation of a patent ductus. |
| 1945 | Crafoord and Nylin publish report of successful coarctation repair in two patients. |
| 1945 | Blalock and Taussig publish report of successful shunts in three tetralogy patients. |
| 1949 | Janeway invites Nadas to develop pediatric cardiology at Boston Children’s Hospital. |
| 1955 | Kirklin and associates report open heart surgery in eight patients with congenital heart disease. |
| 1964 | Mustard reports atrial repair of transposition of the great vessels in a 23-month-old. |
| 1966 | Raskind and Miller report balloon atrial septostomy in three infants. |
| 1966 | Ross and Somerville report homograft repair of pulmonary atresia in an 8-year-old. |
| 1968 | McGoon and associates report repair of truncus in an 8-year-old. |
| 1971 | Fontan and Baudet report successful repair of tricuspid atresia in two of three patients aged 12, 23, and 35 years. |
| 1975 | Jatene and associates report arterial switch for transposition of the great arteries. |
| 1975 | Norwood and associates report successful palliatives of hypoplastic left heart syndrome in two of three patients. Early 1970s M-mode echocardiography begins widespread use. |
| 1975 | Elliot and associates report ductal dilation with prostaglandin E in two patients. |
| 1976 | Bargeron and associates describe axial cineangiography; late 1970s, echocardiography is introduced. |
| 1982 | Kan and associates report percutaneous valvuloplasty for valvular pulmonary stenosis. |
| 1980s-1990s | Doppler studies, color flow, fetal studies, and transesophageal echocardiography become vital part of pediatric cardiology. |
| 1980s | Explosion of studies show possibility and success of percutaneous treatment of pulmonary artery stenosis, coarctation, and aortic stenosis. |
| 1990s | Interventional catheterization therapy for patent ductus arteriosus, pulmonary and aortic stenosis, pulmonary artery stenosis, and many atrial septal defects becomes standard part of management. |
From Graham TP Jr. Minimizing the morbidity of pediatric cardiovascular disease—historical perspective; pediatric cardiology. Prog Pediatr Cardiol 2005; 20:1-6.
For the repair of intracardiac defects, cardiopulmonary bypass was needed, and in 1955, Lillehei3 reported successful repair of ventricular septal defect, atrioventricular septal defect, and tetralogy of Fallot with use of this human cross-circulating technique. Kirklin4 demonstrated the successful use of mechanical cardiopulmonary bypass, reporting eight cases in 1955.
The development of prostaglandins has had an impact on pediatric cardiology and cardiac surgery most significantly. The introduction of prostaglandin E1 in routine clinical use in the mid-1970s5 has allowed proper diagnosis in a timely fashion of a child with congenital heart disease while permitting further clinical stabilization and refinement of the medical management and surgical intervention.
With imaging, cardiac catheterization was a necessary advance for the diagnosis and treatment of congenital cardiac defects, and by the 1950s,6 many centers were routinely studying children with heart defects and planning surgical interventions on the basis of these studies. However, the development of two-dimensional echocardiography and color flow Doppler imaging by the 1980s significantly changed the ability to diagnose infants and children with heart disease and refined the ability of surgeons to perform more complex procedures in infants and young children. Three- and four-dimensional multiplanar echocardiography is a developing imaging modality that is affecting how we visualize intracardiac anatomy and great vessel disease, and it is rapidly becoming an expectation of the surgeon as surgical intervention is planned.
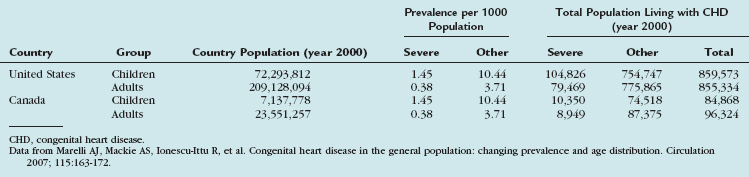
The more intriguing aspect of congenital heart disease is the fact that within the next few years, there will be more adults with congenital heart disease than children with congenital heart disease (Table 28-2).7 Survival to adulthood with a diagnosis of congenital heart disease is now an expectation.
TABLE 28-2 Extrapolated Prevalence of Adult Congenital Heart Disease in the General Population and Extrapolated Numbers to the United States and Canada
This chapter serves as a general overview of congenital heart disease, surgical considerations, and imaging strategies. More detailed aspects of these defects (Table 28-3) are addressed in subsequent chapters.
TABLE 28-3 Relative Frequency of Major Congenital Heart Lesions*
| Lesion | Percentage of all Lesions |
|---|---|
| Ventricular septal defect | 35-30 |
| Atrial septal defect (secundum) | 6-8 |
| Patent ductus arteriosus | 6-8 |
| Coarctation of aorta | 5-7 |
| Tetralogy of Fallot | 5-7 |
| Pulmonary valve stenosis | 5-7 |
| Aortic valve stenosis | 4-7 |
| D-Transposition of great arteries | 3-5 |
| Hypoplastic left ventricle | 1-3 |
| Hypoplastic right ventricle | 1-3 |
| Truncus arteriosus | 1-2 |
| Total anomalous pulmonary venous return | 1-2 |
| Tricuspid atresia | 1-2 |
| Single ventricle | 1-2 |
| Double-outlet right ventricle | 1-2 |
| Others | 5-10 |
* Excluding patent ductus arteriosus in preterm neonates, bicuspid aortic valve, physiologic peripheral pulmonic stenosis, and mitral valve prolapse.
SURGERY FOR ACYANOTIC CONGENITAL HEART LESIONS WITH A SHUNT
Description and Special Anatomic Considerations
Acyanotic congenital heart disease (Table 28-4) is characterized by a lack of cyanosis. In further defining these disorders, they are often classified on the basis of the presence or absence of left-to-right shunt.
TABLE 28-4 Acyanotic Congenital Heart Disease with a Left-to-Right Shunt
Ventricular septal defect (VSD), the most common form of congenital heart disease, represents approximately one third of all major congenital cardiac defects. VSDs are generally classified into one of four groups, depending on their location in the interventricular septum (Fig. 28-1). These may be associated with other cardiac defects, such as atrioventricular valve defects, coarctation of the aorta, and other left-to-right shunts. The ventricular septum anatomy is complex, and many associated anatomic structures are key in the consideration of the repair, such as location of the conduction system of the heart.
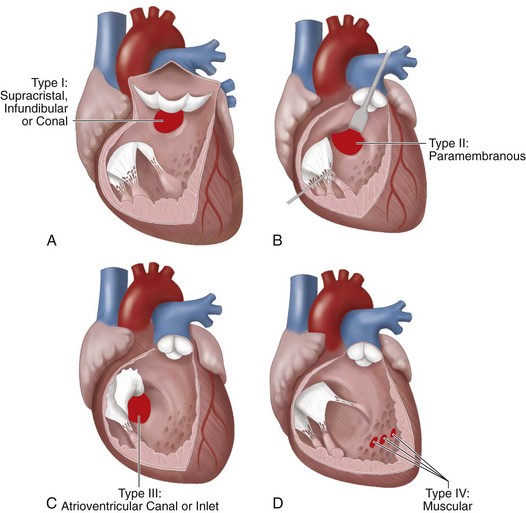
 FIGURE 28-1 The classic anatomic nomenclature assigning VSDs to one of four anatomic types.
FIGURE 28-1 The classic anatomic nomenclature assigning VSDs to one of four anatomic types.
(Redrawn from Wells WJ, Lindesmith GG. Ventricular septal defect. In Arciniegas E [ed]. Pediatric Cardiac Surgery. Chicago, Year Book Medical, 1985.)
Indications
In this broad category of defects, the usual driving indication for early surgical intervention is congestive heart failure and how difficult it is to control with medical management. This is balanced against the surgical risks for the various procedures and the possible comorbidities that may exist as a part of a clinical syndrome or initial clinical presentation. An infant’s weight and gestational age also may play a role in the surgical timing for many of these defects. A typical scenario for surgical intervention based on prematurity and significant lung disease as a result of left-to-right shunt is a patent ductus arteriosus. The advent of indomethacin8 as medical management for closure of these defects has significantly reduced the need for surgical intervention.9 However, in extreme prematurity, renal disease, and severe diastolic “runoff” through a large patent ductus arteriosus, surgery may be a relative emergency.
For example, surgical timing for closure of a hemodynamically significant VSD may be indicated at a few weeks or as late as a few years. The later closure may be indicated for a late-identified supracristal VSD or a perimembranous VSD with a coexistent subaortic membrane or prolapse of the aortic valve leaflets. Both of these defects have been implicated in progressive aortic valve damage,10,11 even though they may be quite small and have no risks for long-term pulmonary vascular disease or congestive heart failure.
With the diagnosis of AVSD, there can be tremendous variability of the surgical timing based on the level of shunting (i.e., atrial vs. ventricular), the complexity of the associated common atrioventricular valve disease, and the associated clinical status (Fig. 28-2).
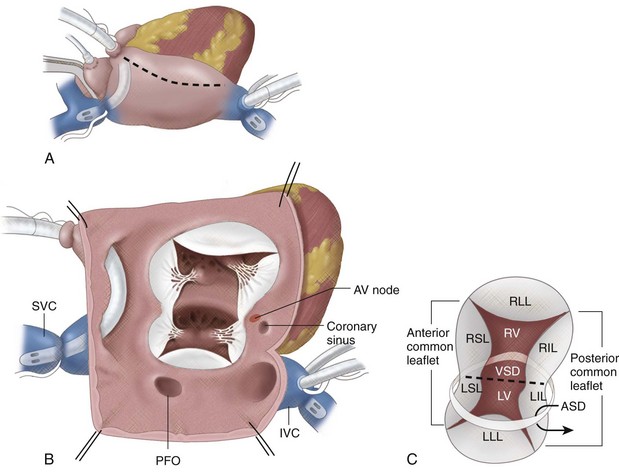
 FIGURE 28-2
FIGURE 28-2


