© Springer International Publishing Switzerland 2015
Ik-Kyung Jang (ed.)Cardiovascular OCT Imaging10.1007/978-3-319-10801-8_1414. Consensus Documents
(1)
CLI Foundation, Rome, Italy
(2)
Department of Surgery, San Giovanni Addolorata Hospital and CLI Foundation, Rome, Italy
(3)
Interventional Cardiology Unit, San Giovanni Addolorata Hospital, Via dell’Amba Aradam 8, Rome, 00184, Italy
Abstract
Optical coherence tomography is a novel technique capable of studying coronary plaques and stented segments with superb resolution. Two consensus documents focused on the clinical applications of OCT for baseline or post-intervention assessment have been released. As the first goal of this chapter, we summarized the conclusion reached by the consensus documents, updating the statements that were made at that time, with new concepts.
The assessment of both atherosclerotic plaque and stent deployment using OCT, and the study of vessel healing require an extensive knowledge of the pertinent definitions. In all three published consensus documents, the authors tried to provide a complete list of definitions needed for conducting appropriate OCT based plaque and stent analyses.
However, researchers still continue to use different definitions especially in cases of plaque erosion and intra-stent thrombus. We first addressed the role of OCT in assessing coronary plaque morphology and vulnerability, then we focused on OCT guidance of coronary interventions.
Keywords
Optical coherence tomographyGuidanceCLIO-PCIStent thrombosisControversyAmbiguous lesion14.1 Current Available OCT Technologies
The main obstacle in the adoption of optical coherence tomography (OCT) imaging in clinical practice is that OCT cannot image through a blood field, and therefore requires clearing or flushing of blood from the lumen [1]. While the previous OCT system called time-domain (TD) OCT, performed by either a vessel occlusion or a non occlusive technique, required a large amount of iso-osmolar contrast [2, 3], the latest technology called frequency-domain (FD) OCT allows us to acquire clear and multiple images simultaneously, reducing the amount of needed contrast.
The acquisition of FD-OCT pullback requires a bolus of crystalloid solution (usually contrast or dextran [3]) injected through the guiding catheter. The quality of OCT images depends on an accurate acquisition technique and proper guiding catheter engagement, a fundamental maneuver to optimizing directional contrast flushing [1].
Currently, there are two FD-OCT catheters on the market: the DragonflyTM© catheter (St. Jude Medical) and the FastView© OCT catheter (Terumo). They have similar functions as they are advanced over a regular guide wire, distal to the region of interest and are provided with a dedicated marker, located 10 mm distal to the OCT lens, which enables the pull-back starting point selection.
The acquisition speed of the DragonflyTM© and FastView© OCT catheters can be set up in a range between 5 and 40 mm/s, enabling the study of vessel segments with lengths of 50 mm or 75 mm. In the former option the system permitted the study of arteries with cross sectional thickness up to 100 μm [1, 4].
In the past decade, intravascular ultrasound (IVUS) was the most used intravascular imaging technique. Unlike IVUS, OCT cannot penetrate the whole plaque thickness; however, OCT can image coronary arteries at a very high resolution (20 μm). Due to the thinner profile of FD-OCT probes, and the faster pull-back imaging speed of OCT, many severely diseased target lesions can be safely imaged without causing luminal obstruction, thus making symptomatic ischemia much less likely [5]. As a main technical drawback, FD-OCT cannot study plaques located at the very ostium of the left or right coronary arteries. Also, in the presence of very tight stenosis, image quality can be sub-optimal [5].
In previous years, TD OCT technology was found to be safe [2, 6, 7], effective [2, 6, 7] and highly reproducible. Later studies carried with the FD OCT technique, confirmed data on the safety and effectiveness and showed that luminal areas and vessel length can be measured with high reproducibility [8–10]. A fair correlation between OCT and IVUS quantitative measurements of the lumen areas was reported [8–10] despite comparative studies showing that IVUS tends to slightly overestimate lumen areas, while stent and neo-intimal areas are slightly higher on OCT [8–10].
14.2 Impact of Plaque Composition on Coronary Intervention
14.2.1 Calcification and Lipid Pool
Identification of calcium can affect an interventional cardiologist’s decision making. In fact, in the presence of circumferential calcification, direct stenting should be avoided and strategy can span from a careful pre-dilatation to test expansion, to cutting balloons for very short calcific rings or to pre-treatment with a rotational atherectomy [11, 12].
Both IVUS and OCT can identify calcium with accuracy [13]. However, IVUS is unable to measure its thickness due to a shadowing effect [14]. Infrared light penetrates calcium better, enabling measurement of superficial calcific components with a thickness less than 1.0–1.3 mm [15, 16]. Only the less common deep intra-plaque calcium deposits cannot be addressed with OCT.
As a relatively new concept stent positioning should be performed by aiming for complete coverage of the lipid pool [17], avoiding the placement of stent edges over lipid formations, that can potentially lead to stent thrombosis and plaque embolization [18]. OCT identifies lipid pools with high accuracy and its pre-intervention use can also be encouraged to reduce the risk of embolization of plaque components.
14.2.2 Assessment of Ambiguous Angiographic Lesions and Deferral of Interventions
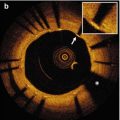
Hazy and intermediate lesions on angiogram are considered a grey-zone for interventional cardiologists [19]. OCT allows detailed analysis of plaque morphology and lumen area, being therefore very instrumental in guiding interventions and helping decide whether to treat coronary narrowings. Unlike other intravascular imaging modalities, OCT has the unique ability to identify thrombi and measure the amount of thrombus. The “OCT defined thrombus score” is a semi-quantitative assessment of a thrombus [20, 21], that can be used in cases of angiographically hazy lesions. These kinds of lesions often appear as ruptured plaques with large amounts of thrombus attached to the site of disruption [19].
Coronary angiograms of patients with acute coronary syndrome may show one or more non-significant lesions, exhibiting only haziness on angiogram. This can pose a problem for the interventional cardiologist. In fact, misinterpretation of culprit lesions may cause ischemic events in the short and mid-term follow-up. Under these circumstances the decision to proceed with treatment rests more on morphologic observations than on the absolute measurement of lumen area.
The fact that lumen contours are easily obtained enables the on-line application of an automated algorithm, that facilitates operator decision making by displaying the reconstructed segment in a longitudinal view.
14.2.3 Identification of Vulnerable Plaques
A number of IVUS studies attempted to characterize the appearance of vulnerable plaque. Recently, the PROSPECT trial, based on a signal radiofrequency analysis of the IVUS backscatter, for the first time showed that intravascular imaging modalities can identify plaque with a higher risk of events at 3 years [22]. OCT, due to its high accuracy in the detection of superficial plaque components, can potentially identify plaque at risk of rupture. In fact, OCT can address many features that have been related to plaque vulnerability such as the presence of thin fibrous cap, extension of lipid pools [5], micro-vessels [23] and inflammatory cells [24]. However dedicated soft-wares, based on the acoustic properties of the OCT signal, are needed to improve identification of the local signs of inflammation [24].
To better understand the change of plaque composition in response to specific treatment, serial studies should be carried out with the specific goal of identifying the same location at different time points. For this task, dedicated three-dimensional soft-wares can be adopted. The recently developed “carpet view software” which unfolds the vessel segment, reconstructing it as an open structure, will likely improve the off-line serial comparisons of target plaques and enable the matching of different imaging modalities [25]. Many experts have suggested that the identification of a vulnerable plaque requires the combination of OCT and other imaging modalities such as IVUS or near infrared spectroscopy to better characterize the plaque and obtain information on deep lesion components [26, 27].
14.3 OCT-Guided PCI Guidance
The main drawback of OCT is its inability, unlike IVUS, to outline the vessel architecture, and measure the external elastic membrane, and the longitudinal extent of plaque burden in lesions with thicknesses exceeding 1.0–1.5 mm. Some interventional cardiologists may argue that measurement of plaque burden has a role in selecting reference segments. Therefore, the pre-intervention use of OCT to select the appropriate stent size and length is mainly based on the assessment of lumen areas. Furthermore, pre-intervention OCT assessment of plaque composition is helpful for predicting the subsequent risk of complications. In fact, calcified and lipid plaques carry an increased risk of stent edge dissections [28], while thin cap fibro-atheroma is more frequently associated with a significant tissue prolapse inside the stent [29].
Recent meta-analysis stressed the role of IVUS in guiding stenting procedures, showing that the rate of stent thrombosis and major adverse clinical outcomes can be significantly reduced [30, 31]. According to these findings, the large IVUS sub-study of the ADAPT-DES registry showed that the presence of attenuated plaque, tissue protrusion, reference segment plaque burden, and edge dissection were significant predictors of stent thrombosis [32]. Lastly, IVUS guidance proved to be useful for treatment of complex lesions such as left main PCI [33]. Due to the improved resolution of OCT, it is reasonable to believe that OCT guidance can also improve the outcome of percutaneous coronary interventions (PCIs). However, the two techniques are different, particularly in their resolutions and penetrations. Therefore, IVUS accepted criteria that require an assessment of vessel architecture, specifically with a measurement of the external elastic membrane and plaque burden, cannot be adopted for OCT use.
On the other hand, OCT can identify details such as stent under-expansion, malapposition, asymmetric stent strut distribution, intra-stent thrombotic formations, and dissections at the edges and inside the stents, with a level of accuracy unmatched by IVUS.
14.3.1 Frequency of OCT Findings of Sub-Optimal Stenting and Clinical Role of OCT Guidance
The multicenter CLI-OPCI study [34], addressed the role of OCT guidance. The registry compared the clinical outcome of 335 patients with OCT guided intervention to those from a control group by means of a propensity score adjustment. OCT guidance was found to improve the 1-year composite event of cardiac death or non-fatal myocardial infarction after PCI in a real-world population. The study also addressed the issue of how to treat OCT findings indicative of suboptimal stent deployment. In 34.7 % of the stented segments, it was determined, based on the OCT results, that further intervention with either balloon dilation (22.3 %) or additional stenting (12.4 %) was needed. The final conclusion of the study was that explicit quantitative OCT thresholds are required in order to improve clinical outcome of patients undergoing PCI.
OCT studies, in patients with acute coronary syndrome, have shown that in-stent tissue protrusion due to the presence of residual thrombus, is a common finding [29]. Recent data revealed that residual intra-stent thrombus is related to peri-procedural myocardial infarction if left untreated [29]. Preliminary data showed that additional OCT-driven in-stent balloon dilatation can significantly reduce the in-stent thrombus area percentage without worsening the microcirculatory indexes [35].
As another crucial application, OCT can clarify mechanisms of restenosis and thrombosis early or late after the index procedure, guiding repeat revascularization and thus minimizing the risk of additional adverse events.
Assessment of stent under-expansion by OCT can be obtained by comparing the minimal stent area with the reference lumen area. Additionally, a threshold of absolute minimum lumen cross-sectional area within the stent could be applied, with an area of at least 5.0–5.5 mm2 previously advocated as the target minimum stent area to prevent failure [36].
A recent study from our group, addressed the incidence of suboptimal OCT results in 21 consecutive patients exhibiting sub-acute thrombosis. The patients were matched 1:2 with a control group of 42 patients from the Rome Heart Research core-lab database [37]. OCT showed that minimum lumen area and minimum stent area measurements were significantly smaller in the stent thrombosis group together with a higher frequency of stent under-expansion, edge dissection and reference lumen narrowing.
Stent follow-up, delayed healing and poor endothelialization are common findings in the pathological specimens of vessels treated with drug eluting stent (DES) [38], and recent post-mortem studies demonstrated that late-stent thrombosis was strongly associated with the ratio of uncovered/total stent struts [39].
Several experimental and clinical studies have demonstrated the high correlation between OCT and histological measurements of tissue coverage of stent struts [40, 41], although OCT is unable to identify the endothelium [5]. In a sub-analysis of the ODESSA trial, 8 % of the stented segments with no detectable neo-intima by IVUS were found to have neo-intimal coverage by OCT [42]. Follow-up OCT data revealed that most drug eluting stents, including those with a biodegradable polymer, were covered with thin neointima, but few revealed complete coverage [42–44]. Incomplete stent apposition without neo-intimal hyperplasia was significantly associated with the presence of an OCT-detected thrombus at the follow-up, and may constitute a potent substrate for late-stent thrombosis [45]; however, so far a subclinical thrombus has not been related to the risk of major adverse cardiac events [46]. Furthermore, incomplete stent apposition and the absence of tissue coverage detected by OCT are more frequently found in the setting of acute coronary syndromes, particularly after DES deployment [47].
Optical coherence tomography can be applied for the treatment of late in-stent restenosis because the strong reflective power of the stent struts allows their detection through thick layers of hyperplasia, allowing optimal sizing of high-pressure balloons to correct under-expansion and facilitating the use of cutting balloons [48].
14.4 Fields of Controversy for OCT Definitions
Although three expert review documents have been published, there is not yet an agreed upon definition among researchers for OCT based plaque and stent analyses. This paragraph aims to highlight the major differences in the definitions used for OCT analyses. OCT can address plaque composition with high accuracy. Lipid-rich plaques have been defined by researchers in several ways: (1) Plaques with lipid material extending for more than 90° [52, 53] or 120° [54, 55] or (2) Lipid plaque with a thin fibrous cap (<400 μm) [56]. A fibrous cap is identified as a signal-rich homogenous band overlying a lipid core [54]. The thickness of the fibrous cap, measured as the distance from the arterial lumen to the luminal border of the lipid pool [56], can be calculated with different methods and there is no general agreement on the number of repeated cap thickness measurements for an accurate value.
Some authors have used a single measure of the thinnest portion of the cap [57], while others have proposed the use of the mean of the three [54, 56, 58, 59] or five [52] separate measurements.
OCT has the ability to identify vulnerable plaque such as thin cap fibro-atheroma (TCFA) which is thought to account for 60–70 % of coronary events [60, 61].
OCT research groups have used different definitions to identify TCFA. TCFA was defined as: (1) plaque with more than 2 lipid quadrants and a cap thickness of less than 65 μm [60]; (2) plaque with more than 1 lipid quadrant and a cap thickness of less than 70 μm [17], or (3) lipid plaque with a cap thickness of less than 80 μm [56]. The comparison with histological studies provided the first [60] and second [17] definitions, whereas an in vivo study of plaque rupture provided the third definition [56].
OCT is also very instrumental in studying the pathophysiology of plaque instability leading to intracoronary thrombus. Complicated plaques can lead to one of the following three different outcomes: plaque rupture, erosion or calcified nodule.
While the presence of plaque rupture can be easily identified by OCT assessment for the presence of fibrous cap disruption and the formation of a cavity inside the plaque [54], there is no agreement on the ability of OCT for identifying erosion and calcified nodules.
Fibrous cap erosion is an endothelial damage or denudation associated with thrombus, but without a clear fibrous cap disruption [58, 62]. Recently a plaque classification algorithm for identifying OCT-defined erosion has been proposed. This approach differentiates definite erosion (superficial thrombus and intact fibrous cap) from probable erosion (either the absence of superficial thrombus and the presence of an irregular surface or large intra-coronary thrombus impairing accurate plaque visualization without associated calcium or lipid in the close proximal and distal cross-sections) [13].
Calcific nodules are considered superficial deposits of calcium that may lead to thrombus formation. OCT has been proposed for identifying these tiny structures, since they appear as nodules protruding into the lumen. These nodules may have an irregular surface [63]. Some authors have identified their prevalence before thrombus formation [14]; others, after fibrous cap rupture and thrombus formation [13].
OCT can investigate strut apposition and coverage and other features indicative of suboptimal deployment, such as edge dissections, thrombus and tissue prolapse.
Two different methods have been used for assessing stent strut malapposition:
1.
Tissue prolapse and intra-stent thrombus are difficult to distinguish. Tissue prolapse is defined as tissue protrusion between stent struts. It has a typical convex-shaped appearance without disruption of the continuity of the luminal vessel surface [69]. It does not extent for more than 250 μm inside the lumen [70]. On the contrary, thrombus is considered an irregular mass protruding for more than 250 μm, that can appear attached to or separate from the stent surface [43].
Stent edge dissection are often imaged by OCT as a disruption of the luminal vessel surface in the edge segments [69] (within 5 mm proximal and distal to the end of the stent) [71, 72]. However, there is no agreement on how to define a significant edge dissection. Some authors have used a dissection angle greater than 60° [73], while others have applied a longitudinal extension greater than >0.67 mm [72].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree