Chapter 4
Gadolinium-Based Contrast Agents
Kimball L. Christianson, Allen W. Song, and Elmar M. Merkle
CASE 1
1. What is the most likely diagnosis?
2. How is the administration of a contrast agent helpful in establishing this diagnosis?
3. How does gadolinium result in increased T1 signal?
4. What is relaxivity?
ANSWERS
CASE 1
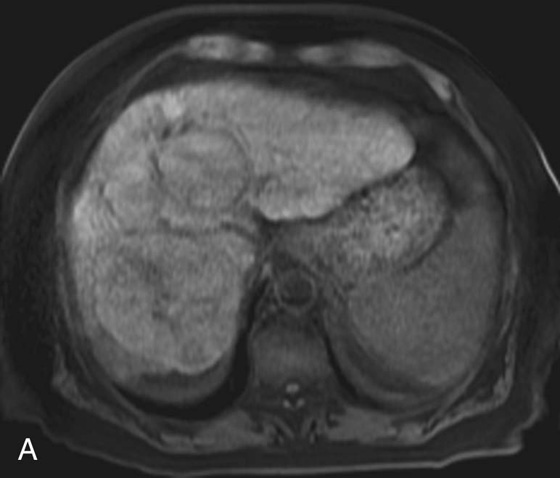
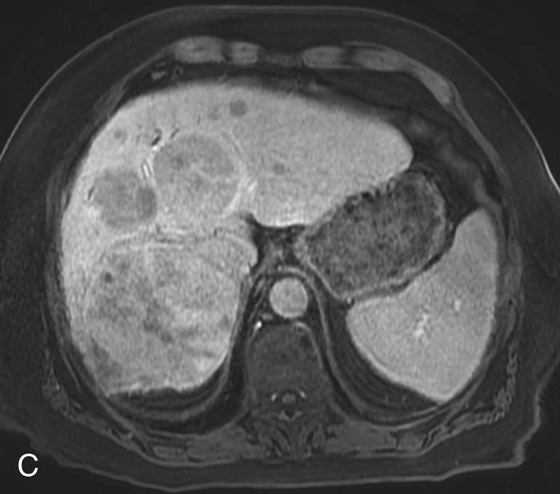
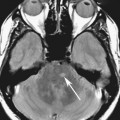
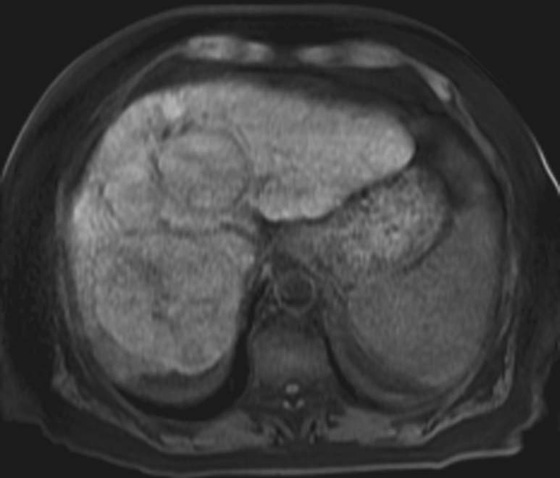
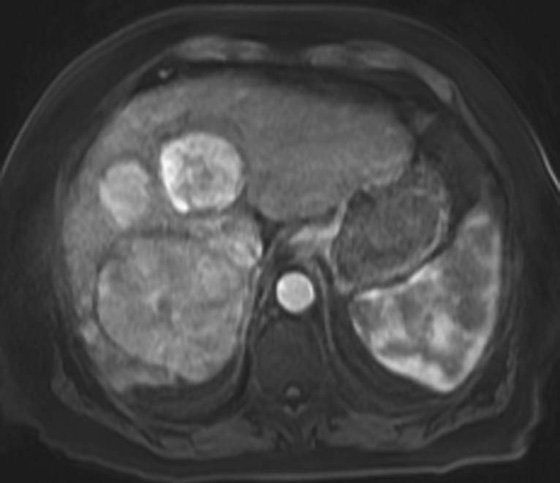
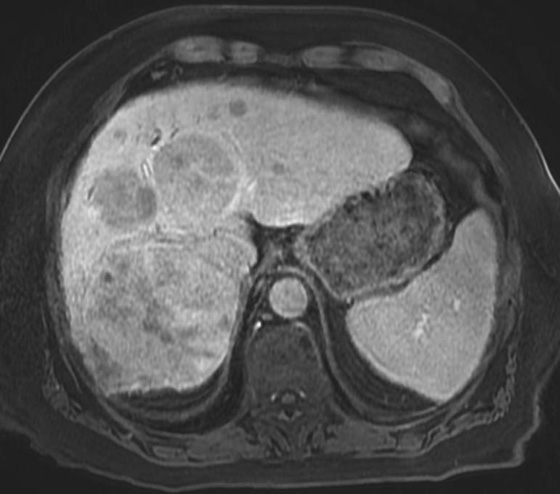
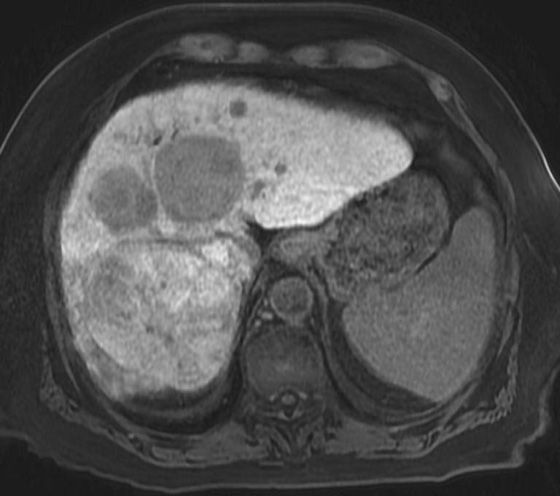
FIGURES 1A, 1B, AND 1C. Fat-suppressed three-dimensional (3D) gradient-recalled echo (GRE) T1-weighted (T1W) precontrast (A) and postcontrast (B and C) images demonstrate hyperenhancement of multiple large liver masses in the arterial phase (B) with rapid washout of contrast seen on the portal venous phase (C).
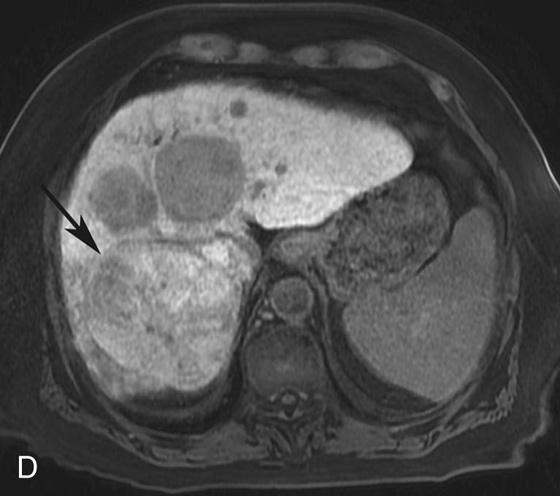
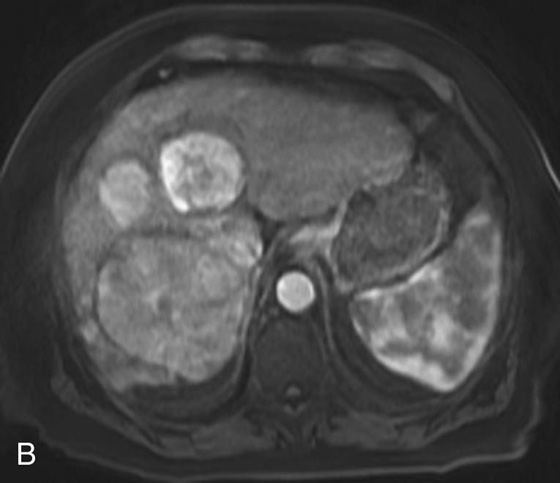
FIGURE 1D. Of note, some tumor components (black arrow) take up the hepatocyte-specific contrast agent gadoxetate disodium, while most of the lesions do not, during the hepatocyte phase.
1. Multifocal hepatocellular carcinoma (HCC).
2. In a cirrhotic patient, a lesion that is rapidly enhancing on the arterial phase with early washout on the later phases is very characteristic of HCC.
3. Gadolinium is a paramagnetic element with seven unpaired electrons. These unpaired electrons spin and create an oscillating magnetic field. When these electrons spin at the proper frequencies, the result is shortening of the T1 relaxation time. Unlike computed tomography, where the iodine molecule itself generates the contrast we see, in magnetic resonance imaging we do not see the gadolinium; we see its effects on the surrounding protons.
4. Relaxivity is a measurement of the efficiency of a contrast agent to shorten T1 and T2 relaxation times.
Diagnosis:
Multifocal HCC in a patient with cirrhosis.
Physics Discussion
Gadolinium is a paramagnetic element that generates a small and positive magnetic field only when placed in an external magnet (e.g., a magnetic resonance imaging [MRI] scanner). This small magnetic field alters the uniformity of the main magnetic field of the scanner to generate useful imaging contrast. Paramagnetic properties arise from unpaired electrons in the element that can realign their orbits in the presence of the external magnetic field. Because gadolinium has seven unpaired electrons, it is highly paramagnetic.
Paramagnetic agents, such as gadolinium chelates, shorten the T1 and T2 relaxation times of adjacent molecules. One important measure of the effectiveness of a contrast agent in shortening relaxation times is relaxivity. In general, the higher the relaxivity of a contrast material, the greater the decrease in proton relaxation time. At low contrast medium concentrations, the T1 relaxation effect predominates as a result of the fast inherent transverse relaxation in tissue. The result is increased signal on T1-weighted (T1W) images. However, at higher concentrations of contrast medium, the T2 relaxation is so short that T2 effects predominate and low signal results.
Paramagnetic gadolinium chelates are believed to increase relaxivity through two primary mechanisms. First, water molecules can transiently bind to the gadolinium chelate and undergo a chemical exchange that enhances relaxation. This is called inner-sphere relaxation. The second mechanism is called outer-sphere relaxation and describes the enhanced relaxation created by the interaction of the surrounding protons with the dipole moment created by the paramagneticion.1
The relaxivity of a contrast agent varies by magnetic field strength. The relaxivity of contrast agents decreases as the magnetic field strength increases (Table 1).2
Table 4-1 Relaxivities of Common Commercially Available MR Contrast Agents
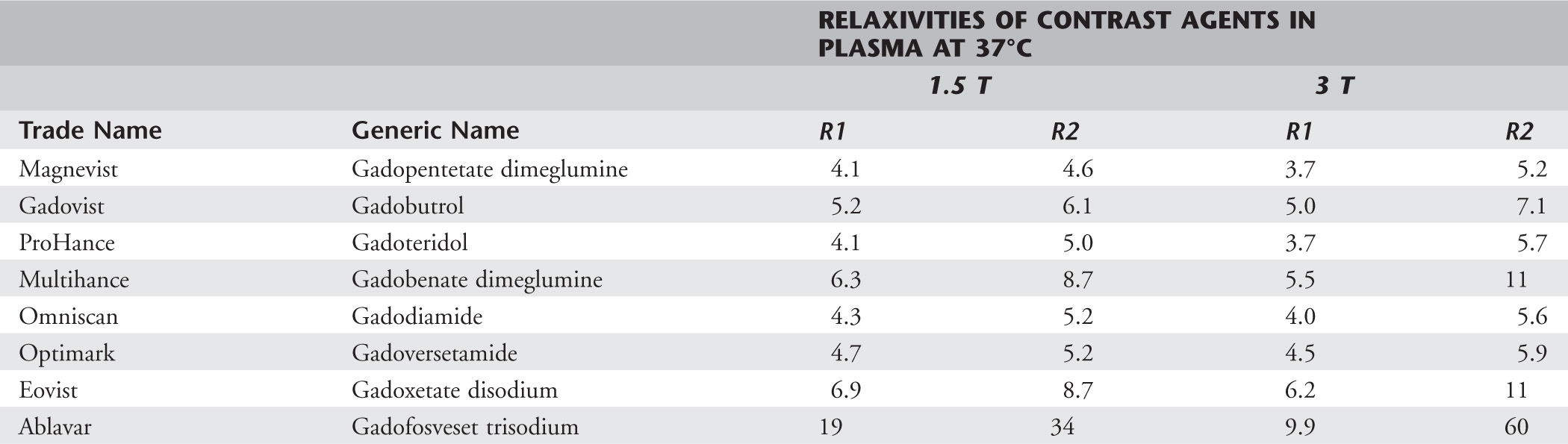
Adapted with permission from Rohrer M, Bauer H, Mintorovitch J, et al: Comparison of magnetic properties of MRI contrast media solutions at different magnetic field strengths. Invest Radiol 40:715-724, 2005.
Contrast Agents
Contrast agents can be divided into extracellular agents, agents that are both extracellular and hepatobiliary, and blood pool agents.
Extracellular Agents
These are the most commonly used and least expensive magnetic resonance (MR) contrast agents. The most common trade names include Magnevist, Omniscan, ProHance, Optimark, and Gadovist. Gadolinium chelates have pharmacokinetics similar to iodine. Excretion is almost entirely through the kidneys.
Combined Agents
These are contrast agents that demonstrate both extracellular and hepatobiliary behavior. Examples include Multihance and Eovist.
Multihance has several important differences from the purely extracellular gadolinium chelates. First, Multihance demonstrates weak binding to plasma proteins, resulting in prolonged time within the intravascular space. Prolonged intravascular time allows for more T1 shortening to take place and in turn allows for lower doses to be administered while achieving the same T1 shortening. This fact has made Multihance the default agent for contrast-enhanced MR angiography (MRA) for many institutions (please note: this is an off-label indication!). Second, hepatocytes take up 2% to 5% of Multihance through an organic anionic transport mechanism otherwise used by bile salts, organic anions, and bilirubin.3 Finally, the hepatocytes excrete Multihance into the biliary canaliculi through the canalicular multispecific organic anionic transport mechanism (cMOAT; aka multidrug-resistance–associated protein [MRP]). The hepatocyte uptake allows for the acquisition of T1W datasets during the hepatocyte phase, which usually takes place 40 to 120 minutes postinjection.
Eovist is another paramagnetic contrast agent with both extracellular and hepatobiliary excretion. In contrast to Multihance, 50% of Eovist is excreted into the biliary system and 50% is excreted through the kidneys. Like Multihance, Eovist binds weakly to plasma proteins primarily through the addition of the EOB ligand, which decreases its tumbling rate, resulting in increased time within the blood pool, higher relaxivity, and a lower administered dose if desired. Because of the markedly higher hepatocyte uptake, the acquisition of hepatocyte-phase images and/or T1W MR cholangiography datasets can be performed much earlier, usually within the first 20 minutes postinjection. This has significant implications on patient workflow as the patient does not need to come back to the MR suite for delayed imaging.
Blood Pool Agents
Ablavar is currently the only contrast agent that is U.S. Food and Drug Administration (FDA) approved for contrast-enhanced MRA. It has several properties that make it unique. The extracellular contrast agents have short plasma half-lives (about 80 to 100 minutes). Ablavar is unique in that it binds reversibly to albumin at a high rate, enabling the contrast agent to remain in the intravascular space for a much longer period (plasma half-life about 15.5 hours). The relaxivity of Ablavar is about four times higher at 1.5T when compared to extracellular contrast agents, allowing a much smaller dose to be administered. Use of the other contrast agents in contrast-enhanced MRA depends heavily on perfectly timing the arterial phase. Ablavar potentially allows for increased flexibility in imaging the vasculature because its vascular contrast lasts for approximately 1 hour.4
Adverse Effects of Contrast Agents
Unbound gadolinium is toxic. This is in part related to the fact that the gadolinium ion (Gd3+) is roughly the same size as the calcium ion and can act as an antagonist at calcium receptors in the body. This can have a deleterious effect wherever calcium plays an important role, such as in respiration and muscle contraction. It can also result in deleterious effects to the liver and spleen and act in enzyme inhibition. The chelates that are bound to gadolinium significantly reduce its toxicity.
Nephrogenic Systemic Fibrosis
Nephrogenic systemic fibrosis (NSF) is a rare fibrosing disorder that appears to be associated with renal failure and gadolinium use. Clinical symptoms of NSF include swelling and eventual induration and fibrosis of the lower extremities, upper extremities, and lower abdomen. In severe cases the vital organs and muscles can become involved, which can be life threatening. As was previously mentioned, free Gd3+ is highly toxic. There may be an association between free Gd3+ and the development of NSF. In order to avoid the toxic effects, Gd3+ water-soluble chelates were made that demonstrate high affinity for the Gd3+ cation.
Stability describes the tendency of a Gd3+ cation to remain bound to its chelates. Stable chelates characteristically are ionic and have a cyclic morphologic structure. Conversely, chelates that are nonionic and have a linear configuration are less stable. In terms of stability, the configuration of the molecule is more important than the ionic state. The vast majority of cases of NSF have been reported with Omniscan, which is nonionic and has a linear morphologic structure (Table 2).5,6
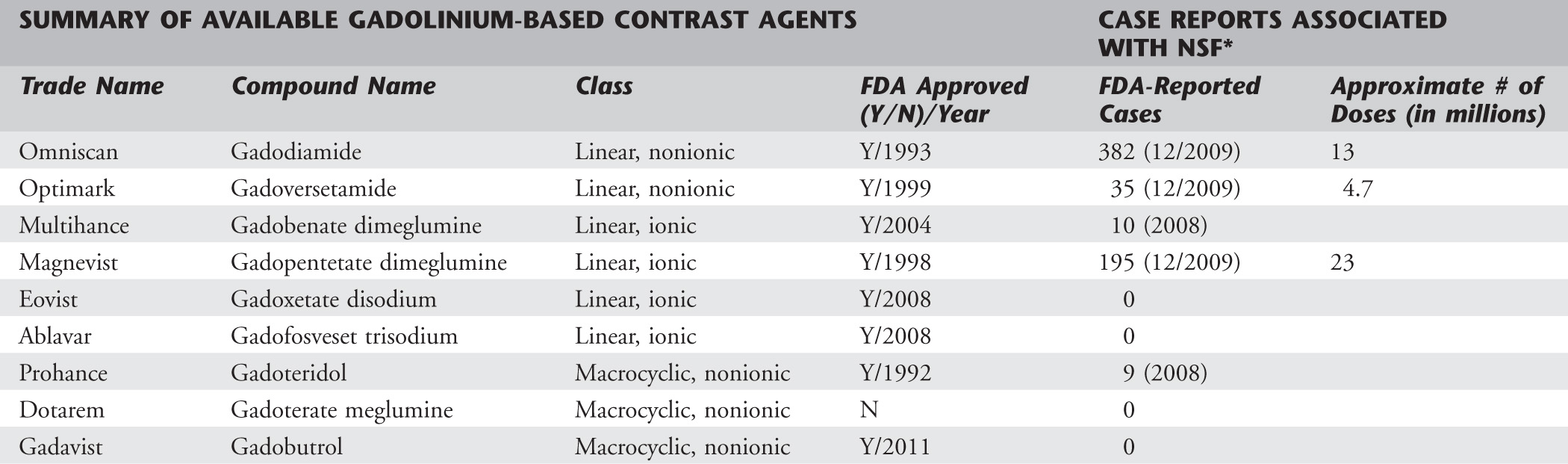
*The most current data within the last two columns comes from the ACR Manual on Contrast Media, Version 7, 2010, which specifically mentions the number of FDA-reported cases associated with Omniscan, Optimark, and Magnevist as of December, 2009.
Data from Juluru K, Vogel-Claussen J, Macura K, et al: MR imaging in patients at risk for developing nephrogenic systemic fibrosis: protocols, practices, and imaging techniques to maximize patient safety. RadioGraphics 29:9-22, 2009; and Penfield J, Reilly R: Nephrogenic systemic fibrosis risk: is there a difference between gadolinium-based contrast agents? Semin Dialysis 21:129-134, 2008.
Current risk factors for the development of NSF include intravenous administration of gadolinium-based contrast agents (GBCAs) in patients with acute and chronic renal failure.5 The risk of developing NSF is increased with higher doses of contrast. Renal failure is hypothesized to increase toxicity by two mechanisms. First, the delay in excretion of gadolinium chelates due to renal failure results in prolonged retention in the body. This increases the chance that the gadolinium chelates will dissociate and release free Gd3+. The second mechanism is referred to as transmetallation. Transmetallation is a process by which another metal in the body replaces the chelated Gd3+ cation and free Gd3+ molecule is released.7
In order to minimize the risk of NSF related to exposure to GBCAs, the FDA recently issued the following recommendations8:
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree