Based on their predominant relaxivity effects, GBCM can be classified as paramagnetic or superparamagnetic.
Paramagnetic or relaxation agents (also called positive contrast agents) have relatively large numbers of unpaired electrons. These unpaired electrons generate a locally fluctuating magnetic field, which cause a shortening in relaxation time T1 and induce a positive enhancement of the signal in T1-weighted images. The effect on T2-weighted images is present but less evident than in T1-weighted images. The magnetic momentum is nulled when the paramagnetic substance is removed from the magnetic field. The most utilized paramagnetic substances are the lanthanides, among which the most used is gadolinium. Gadolinium chelates are the most CA used for MR angiography because of their shortening of the T1 relaxation of blood (bright blood sequence).
Superparamagnetic or susceptibility agents (also called negative contrast agents) develop large magnetic moments when placed into a magnetic field much greater than those seen with paramagnetic agents such as gadolinium [8] and determine a local lack of homogeneity in the magnetic field at a macroscopic level which induces a marked fluctuation of the magnetic momentum between the blood and intracellular compartments, causing a shortening of T2* of the neighboring 1H nuclei, thus determining a decrease in signal. Two types of compounds are discriminated based on their aggregate size: large (>50 nm) particles, referred to as a SPIO (superparamagnetic iron oxide) and small (<50 nm) particles, referred to as a USPIO (ultrasmall superparamagnetic iron oxide). Examples of SPIOs include AMI-25 and SHU-555A; USPIOs include AMI-227 and NC100150. Superparamagnetic contrast agents have been proposed for studies on the cardiovascular system with satisfying results both in animal and human studies [9, 10].
3 Pharmacokinetic
Contrast agent’s pharmacokinetic knowledge is necessary before approaching a carotid MRA. The principal characteristics are resumed in Table 5.1.
Table 5.1
Physiochemical properties of interstitial GBCM
Genetic name | Product name | Manufacturer | Chemical abbreviation | R1a | R2a | Molarity | Osmolality | Viscosity |
|---|---|---|---|---|---|---|---|---|
Gadopentetate | Magnevist | Berlex | Gd-DTPA | 3.8 | 0.500 | 1.96 | 2.90 | |
Gadoversetamide | MultiHance | Bracco | Gd-BOPTA | 4.4 | 5.6 | 0.500 | 1.97 | 5.30 |
9.7 | ||||||||
Gadodutrol | Gadovist | Bayer | Gd-DO3A-butrol | 3.6 | 1.000 | 1.39 | 3.70 | |
Gadoterate | Dotarem | Guerbet | Gd-DOTA | 3.6 | 4.8 | 0.500 | 1.35 | 2.00 |
Gadoteridol | ProHance | Bracco | Gd-HPDO3A | 3.7 | 0.500 | 0.63 | 1.30 | |
Gadodiamide | Omniscan | Nycomed, Amersham | Gd-DTPA-BMA | 3.9 | 0.500 | 0.79 | 1.40 |
Molarity is defined as the amount of a constituent divided by the volume of solvent. MR contrast agents must be formulated at adequate concentration to effectively alter relaxation at achievable blood concentrations and must be either soluble or homogeneously dispersible in blood. The molarity of CA employed are 0.1–1.0 M depending on agent relaxivity (Tables 5.1 and 5.2).
Table 5.2
Physiochemical properties of blood pool GBCM
Genetic name
Product name
Manufacturer
Chemical abbreviation
R1a
R2a
Molarity
Osmolality
Viscosity
Gadofosveset
Vasovist
Shering
MS-325
6.6
53.0
0.250
44.0
B22956
Bracco
B22956
6.5
0.250
27.0
SHL 643
Gadomer-17
Bayer
Gadomer-17
18.7
0.500
P792
P792
Guerbet
P792
39.0
0.035
44.5
Viscosity measures the fluid resistance and must be low enough that bolus injection can be performed through intravenous (IV) catheters. Warming the contrast agent to body temperature reduces viscosity, and it is useful when injecting high-viscosity agents such as gadopentetate dimeglumine through smaller IVs [24, 25]. Recently, some authors report viscosity as a risk factor for contrast-induced nephropathy (CIN) [11].
Osmolality is the measure of solute concentration. Acceptable contrast agent osmolalities range from 0.3 to 2.0 Osm/kg [7].
The biodistribution of GBCMs is governed by the kinetics of distribution, clearance, and excretion. Simplifying kinetics is possible to identify two compartments with an intravascular space (arteries and veins) and extracellular extravascular space (EES). After injection, contrast media pass from the intravascular space into the EES due to the high intravascular concentration; this phenomenon takes variable time: extracellular fluid agents (ECF) agents diffuse rapidly; blood pool agents and SPIOs diffuse slowly. This process is measured by distribution half-life T1/2 that represents the time required for half of contrast to redistribute from the intravascular space to the EES. Elimination pathways vary according to the type of contrast agent. The gadolinium ECF agents, small and extremely hydrophilic, are predominately eliminated unmetabolized via a renal pathway (glomerular filtration, active secretion, or a combination of the two). The elimination half-life T1/2 represents the time required for elimination of one-half the total contrast from the body, and it is on the order of 90 min for conventional contrast media. The only exception to pure renal elimination is gadobenate dimeglumine (Gd-BOPTA), which is partially taken up by the hepatocytes and excreted in bile [12].
Based on GBCM biodistribution, it is possible to identify two class of molecules:
Extracellular fluid agents (ECF) or parenchymal agent (PA) contain one gadolinium ion (see Fig. 5.1) and are quite small (<1,000 Da) so their distribution half-life T1/2 is low. Characteristics of PA are resumed in Table 5.1 and PA biodistribution is described in Fig. 5.2.
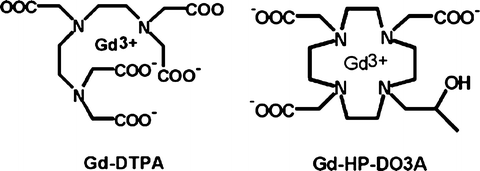
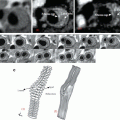
Fig. 5.1
Molecular structure of two GBCMs that differ for how Gd is chelated inside the molecule; the shell-like structure of the molecule avoids any contact between the Gd ion and the tissue components
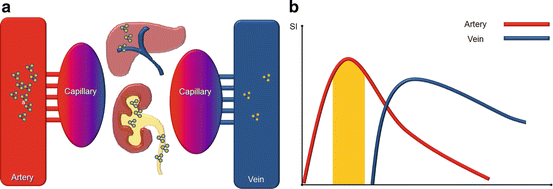
Fig. 5.2
Biodistribution (a) and effects on blood signal intensity (b) of interstitial GBMC. The initial arterial concentration of interstitial GBCM rapidly decreases owing to renal and, later on, hepatic excretion (in smaller percentage). This allows to acquire images only during a short time window (yellow area under the curve) corresponding to the peak of arterial enhancement (Color figure online)
Intravascular contrast agent (ICA) or blood pool agents (BP) remain intravascular for a much longer time and are eliminated from the body much more slowly (longer T1/2) as you can see in Fig. 5.3. These agents remain largely intravascular due to one of two mechanisms: (a) a substantial fraction (>80% at any one time) reversibly binds to serum albumin or (b) the large inherent size (>20,000 Da) of the blood pool agent prevents it from diffusing through the endothelium. This particular property led to steady state sequence acquisition; these are particularly high definition sequence, acquired after traditional first pass sequence, in order to characterize plaque morphology. Intravascular CA are out of commerce or not yet approved by FDA or UE, so some authors [5] tried to acquire steady state sequence also with interstitial GBCM with good to excellent results. See Table 5.2 for BP agents’ properties.
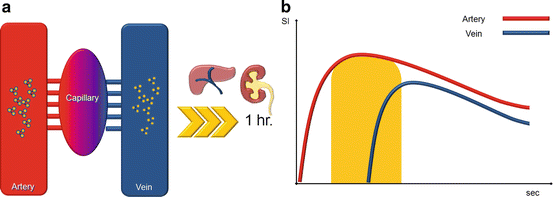
Fig. 5.3
Biodistribution (a) and effects on blood signal intensity (b) of blood pool GBMC. The arterial concentration of interstitial GBCM remains constant in time, since its interstitial filtration is minimal and the renal and hepatic excretion occur later (vascular half life of 1 h) as compared to conventional molecules. This allows to acquire images during a longer time window (yellow area under the curve), even if veins are enhanced, since the increased spatial resolution allowed by longer angiographic sequences reduces artery/veins superimposition issues, allowing at the same time the evaluation of smaller anatomic details that cannot be appreciated with conventional sequences used for arterial images with interstitial GBCM (Color figure online)
4 Safety
Historically, GBCMs are considered to have less adverse reactions than the other contrast media and no nephrotoxicity (at approved dosages for MR imaging). For several years MR with gadolinium has been used to replace contrast-enhanced CT in patients at risk for developing renal failure if exposed to iodinated contrast media. However, in the nephrogenic systemic fibrosis (NSF) era, this practice should only be considered after reviewing the recommendations for use of gadolinium-based contrast in this group of patients. Gadolinium chelates have been approved for parenteral use since the late 1980s, and they are extremely well tolerated by the vast majority of patients in whom they are injected. Acute adverse reactions are encountered with a lower frequency than is observed after administration of iodinated contrast media; the frequency of all acute adverse events after an injection of 0.1 or 0.2 mmol/kg of gadolinium chelate ranges from 0.07 to 2.4% [13]. The majority of these reactions are mild, including coldness at the injection site, nausea with or without vomiting, headache, warmth or pain at the injection site, paresthesias, dizziness, and itching. Reactions resembling an “allergic” response (rash, hives, urticaria, and bronchospasm) are very unusual (0.004–0.7%), and severe reactions (life-threatening anaphylactoid or nonallergic anaphylactic reactions) are exceedingly rare (0.001–0.01%). Fatal reactions to gadolinium chelate agents occur but are extremely rare. Symptoms and treatments are resumed in Table 5.3. Literature [14, 15] reports some risk factors for adverse reaction as:
Table 5.3




Classification of severity and manifestations of adverse reactions to contrast media
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree