Description of Coronary Angiography
Invasive coronary angiography (CA) remains the standard for identifying coronary artery narrowings related to coronary artery disease (CAD). Selective CA, which was first performed by F. Mason Sones in 1959, enables correlation of different clinical syndromes and electrocardiography (ECG) findings with coronary artery anatomy. The method provides the most reliable information for determining appropriate therapy in patients with CAD. CA has become a routine procedure performed on an ambulatory basis in many centers. Technical and logistic improvements have improved patient safety.
The physical requirements for invasive CA performed in a catheterization laboratory include a radiographic system, equipment for physiologic data monitoring and acquisition instrumentation, and equipment for emergency patient care. High-resolution x-ray imaging is required for optimal visualization of the coronary tree, including arterial side branches. Traditional film-based cineangiography has in large part been replaced by digital technology. The digital technique enables immediate online review, quantitative computer analysis, image manipulation capabilities, and increased storage capabilities. During the last few years, direct digital imaging with flat panel technique has become the standard in many catheterization laboratories.
Angiography is performed under local anesthesia with small-diameter catheters introduced through a transarterial sheath. The outer diameter of the catheter is specified using French units, where one French unit (F) = 0.33 mm. Normally, 4- or 5-F catheters are used for diagnostic purposes. In the majority of cases, either the femoral or the radial route is used. Through the catheters, which are introduced over a guide wire, iodinated contrast media is injected selectively into the left and right coronary arteries. A few angiographic projections (varying degrees of left, right, cranial, and caudal angulation) are used to enable visualization of the whole coronary tree without superimposition of multiple vessels.
Indications and Contraindications
CA is used to establish or rule out the presence of coronary stenoses, define therapeutic options, and determine prognosis. CA is also used as a research tool for follow-up after invasive procedures or pharmacologic therapy. The American College of Cardiology/American Heart Association (ACC/AHA) Task Force and the European Society of Cardiology established the indications for CA.1,2 Patients with suspected CAD who have severe stable symptoms and those with certain high-risk features for an adverse outcome should have CA. High-risk criteria include low ejection fraction and poor exercise capacity on an exercise test.
In patients with non–ST-segment elevation acute coronary syndromes (unstable angina and myocardial infarction) with high-risk features (eg, ongoing ischemia, heart failure), CA is recommended during the hospitalization.3,4 In patients with acute ST-segment elevation myocardial infarction (STEMI), guidelines recommend CA in the acute phase for most patients.5,6 Primary percutaneous intervention (PCI) is usually performed in the same procedure, immediately after the diagnostic CA, in these patients.
There are no absolute contraindications for CA. Relative contraindications include febrile untreated infection, severe anemia, severe electrolyte imbalance, active bleeding, acute renal failure, and ongoing stroke. Risk factors for significant complications after CA include advanced age, morbid obesity, bleeding diathesis, recent stroke, and dissection or severe atheromatosis of the thoracic aorta.
Complications
With modern technical equipment, major complications are uncommon (<1%) in diagnostic procedures.7 Vascular complications related to the arterial puncture site are the most frequent complications. Mortality risk is 0.1% or less. Allergic contrast reactions, worsening kidney function, and cerebrovascular accidents are rare complications. Ventricular fibrillation may be provoked by contrast injection into a small side branch, typically the conal branch of the right coronary artery (RCA). Iatrogenic coronary artery dissection is a potential life-threatening complication, which usually is handled by either emergent coronary artery stenting or bypass surgery.
Coronary Anatomy and Variations
The Coronary Artery Surgery Study (CASS) investigators established the nomenclature that is most often used for coronary artery description.8 Minor modifications to the criteria were provided by the Bypass Angioplasty Revascularization Investigators (BARI).9 The coronary artery circulation is composed of two principal arteries, the left coronary artery (LCA) and the RCA, arising from the aorta. The two principal coronary arteries and their larger branches are arranged on the surface of the heart (extramural vessels) and give rise to branches that penetrate the myocardium (intramural vessels). The major epicardial vessels and their second- and third-order branches can be visualized by CA. The network of smaller intramyocardial branches generally is not seen. Not infrequently, the extramural vessels may be covered in part by myocardium and surrounded by cardiac muscle (termed myocardial bridging; see later section “Bridging“). Variations in the branching pattern are extremely common in the human heart. According to the BARI classification, the RCA is predominant in approximately 85% of individuals, providing the posterior descending (PD; ie, posterior interventricular) branch and at least one posterolateral (PL) branch (Figs. 4–1, Figs. 4–2, 4–3).9 In 7% to 8% of individuals, the coronary circulation is left-dominant; the PL, the PD, and the atrioventricular (AV) nodal branches are all supplied by the terminal portion of the left circumflex coronary artery (LCx; Figs. 4–4 and 4–5). In another 7% to 8% of hearts, there is a codominant or balanced system, in which the RCA gives rise to the PD branch and the LCx gives rise to all the PL branches and, in some individuals, also to a parallel PD branch that supplies part of the interventricular septum (Figs. 4–6 and 4–7).
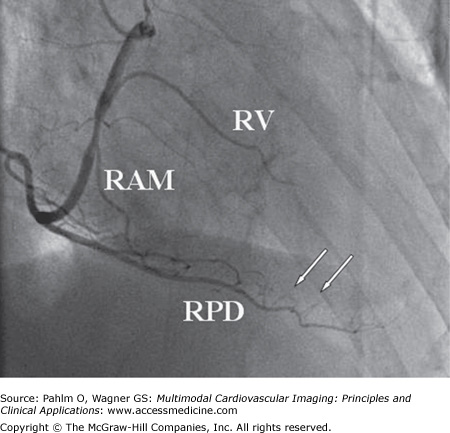
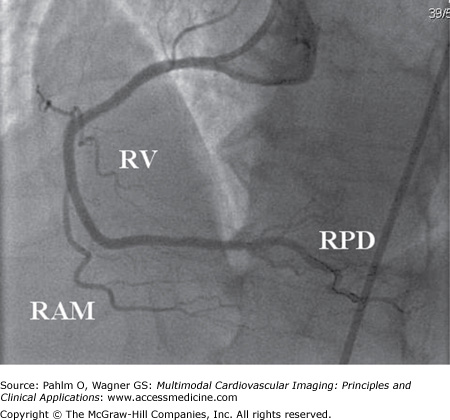
Figure 4–1.
Right anterior oblique projection of the right coronary artery in right dominant circulation. The large posterior descending (PD) branch subtends the inferior part of the heart giving off small inferoseptal branches (white arrows). In this case, the PD branch reaches the left ventricular apex. A borderline significant stenosis is located at the take-off of the right ventricular (RV) and right acute marginal (RAM) branches. Total occlusion of the artery at this point would result in right ventricular transmural ischemia with ST-segment elevation in the right-sided electrocardiogram leads (V3R-V5R) and less often in lead V1. RPD, right posterior descending branch.
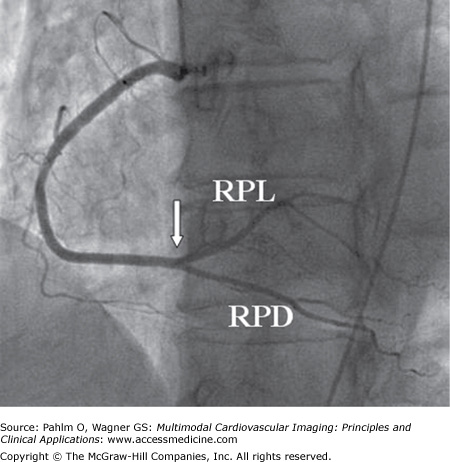
Figure 4–2.
Left anterior oblique projection of the right coronary artery (RCA) in right dominant circulation. At the crux cordis (white arrow), the point where the atrioventricular and interventricular grooves converge, the artery bifurcates into the posterior descending (PD) and posterolateral (PL) branches. The PD branch runs along the interventricular groove, whereas the PL branches spread out over the infero (postero) lateral aspect of the heart. RPD, right posterior descending branch; RPL, right posterolateral branch.
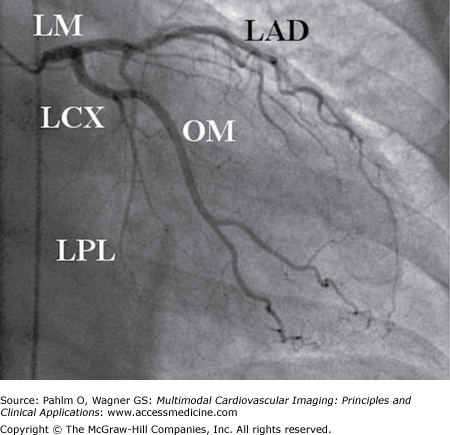
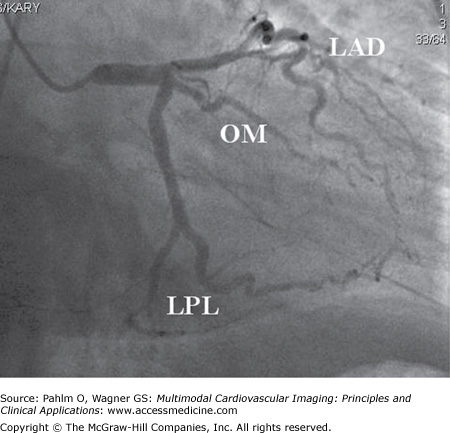
Figure 4–3.
Right anterior oblique caudal projection showing the left coronary artery in right dominant circulation. The left main coronary artery (LM) bifurcates into the two main left-sided epicardial coronary arteries, the left anterior descending (LAD) artery and the left circumflex (LCx) artery. The LAD extends to the left ventricular (LV) apex but does not reach the inferior part of the heart because the patient has a big posterior descending branch of the right coronary artery (RCA). Occlusion of the LAD in this patient would not result in ST-segment elevations in the inferior leads II, III, and aVF. The LCx divides into a large obtuse marginal (OM) branch subtending the anterolateral part of the LV and small posterolateral (PL) branches. The infero (postero) lateral part of the LV is subtended by the RCA. In this patient, occlusion of the proximal part of the LCx would result in a lateral ST-segment elevation myocardial infarction because of the anatomy (small PL branches, large OM branch). The electrocardiography findings would be practically identical if the occlusion were in the OM branch. LPL, left posterolateral branch.
Figure 4–4.
In left dominant circulation (left anterior oblique projection), the right coronary artery is small, giving off right ventricular (RV) and right acute marginal branches, which supply the RV almost exclusively. Occlusion of this type of arteries results in ST-segment elevation in the right precordial leads V1 and V2 (sometimes V3) and reciprocal ST-segment depression in lead V6. The electrocardiography findings may be misinterpreted as anterior ST-segment elevation myocardial infarction caused by left anterior descending artery occlusion. Due to the absence of contralateral ischemia (no cancellation of injury vectors), the ST elevations may be quite impressive despite the limited area of ischemia.
Figure 4–5.
In left dominant circulation (left anterior oblique cranial projection), the left anterior descending (LAD) artery runs along the anterior interventricular groove, reaches the apex, and wraps around the apex into the inferior interventricular groove. A distal occlusion of this artery would result in inferior ST-segment elevation myocardial infarction: ST-segment elevation in leads II, III, and aVF and reciprocal ST-segment depression in leads aVL and I. The left circumflex artery is also big, giving off obtuse marginal (OM) and left posterolateral (LPL) branches and a left posterior descending (LPD) branch.
The proximal or main segment of the LCA is known as the left main (LM) coronary artery (see Fig. 4–3). The left anterior descending (LAD) coronary artery is a direct continuation of the main trunk; it runs along the anterior interventricular groove (see Fig. 4–5). It may terminate before the left ventricular (LV) apex or at the apex (see Fig. 4–3). In most cases, the LAD “wraps” around the apex into the posterior interventricular groove (see Fig. 4–5). One or more left diagonal (LD) branches arise from the LAD, subtending the anterolateral part of the LV (Fig. 4–8). The LAD also gives rise to approximately 10 septal branches (Fig. 4–9; see also Fig. 4–8). One of the proximal septal branches is usually larger than the others and supplies the region of the bundle of His and bundle branches of the conduction system (see Fig. 4–9). The LCx arises from the LM and gives off branches to the upper lateral LV wall and the left atrium (see Fig. 4–3). In approximately two-thirds of cases, the LCx terminates between the lateral margin of the LV and the posterior interventricular sulcus (Fig. 4–10). The left obtuse marginal (OM) branches arise at a right or an acute angle from the LCx and descends vertically toward the apex of the heart (see Fig. 4–3). In approximately one-third of individuals, the LM trifurcates; the intermediate (IM) branch (ramus intermedius) comes off between the LAD and the LCx (see Fig. 4–9). The direct origin of the LAD and the LCx by separate ostia from the aorta without an LM coronary artery is a rare occurrence.
Figure 4–8.
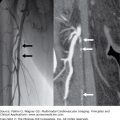
Contrast injection into the right coronary artery (right anterior oblique projection) shows well developed (Rentrop grade 3) collateral channels (arrows) from the right posterior descending (RPD) branch to the chronically occluded left anterior descending (LAD) artery. A small left diagonal (LD) branch and some septal branches of the LAD also fill retrogradely.
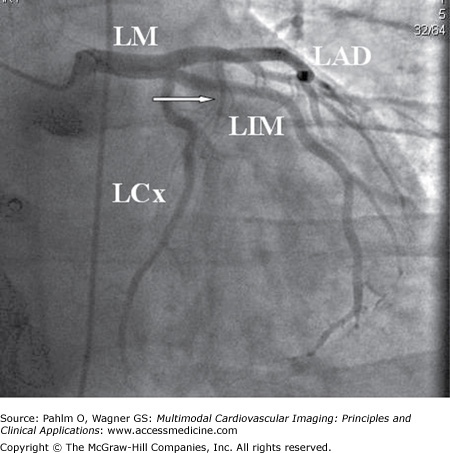
Figure 4–9.
Left coronary artery in caudal projection. The left coronary artery trifurcates into the left anterior descending (LAD) artery, the left circumflex (LCx) artery, and the left intermediate (LIM) artery. In patients with a large intermediate branch, the LCx typically has no or only minor obtuse marginal branches. The large first septal branch is indicated (arrow). LM, left main artery.
Figure 4–10.
Left anterior oblique caudal (“spider”) projection of the left coronary artery. The level of occlusion in the left circumflex (LCx) artery is critical for determining whether the electrocardiogram (ECG) will show ST-segment elevation myocardial infarction (STEMI) or non–ST-segment elevation myocardial infarction. In proximal occlusion, before the obtuse marginal (OM) branch, STEMI will be present. If the occlusion is distal to the obtuse margin of the heart (two-headed arrow) (distal to the OM branch), the ECG shows ST-segment depression or is without significant ST-segment changes in the 12-lead ECG. LM, left main artery; LPL, left posterolateral branch.
The RCA usually gives rise to a large branch along the acute margin (RAM branch) of the heart (see Fig. 4–1). In most individuals (right dominance), the RCA gives off the PL and PD branches at the crux cordis (see Fig. 4–2). The AV nodal branch arises from the PL branch. The most proximal side branch, the conus branch, subtends the right part of the interventricular septum to a varying extent (Fig. 4–11). In approximately 50% of individuals, the conus branch takes off directly from the aorta, either through a separate ostium (two-thirds of individuals) or through a common ostium with the RCA (one-third of individuals). The branch to the sinus node arises from the proximal RCA in the majority of individuals (see Fig. 4–11). In approximately 45% of human hearts, the sinus node is supplied by a branch arising within the first few millimeters of the course of the LCx.