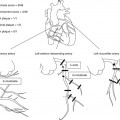
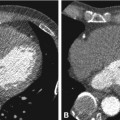
Fig. 22.1
Development of the coronary arteries. Vasculogenesis includes the in situ formation of a primitive capillary plexus in the subepicardial space from immature endothelial precursors partly originating from the proepicardial organ (PEO in Panel a). This is also the phase during which early differentiation into arterial (red) and venous (blue) endothelium occurs. Angiogenesis is initiated by the formation of a vascular network via transmyocardial sprouting from preexisting vessels and intussusception, i.e., formation of new pillars and septations within the vascular sprouts (Panel b). Subsequent embryonal arteriogenesis begins with the connection of the network to the aorta and continues with extensive remodeling, formation of distinct vascular provinces, recruitment of smooth muscle cells, and vascular stabilization (Panel c). For details on arteriogenesis of the coronaries see Fig. 22.2 (Modified from Y. von Kodolitsch et al. Zeitschrift für Kardiologie 2004)
In the initial stage of embryonic development of the heart, the endocardium and myocardium are being nourished by the blood flowing through the lumen of the heart tube. By the beginning of the third week, as the walls of the developing heart become thicker, diffusion is no longer sufficient to supply the heart tube. At this stage endothelial precursor cells migrate into the myocardium and commence forming the primordial vascular structures (vasculogenesis). The initial channels formed within the epicardial covering are scattered throughout the myocardium, inducing an epicardial-to-endocardial vascular gradient, regulated among others by ventricular epicardial growth factor.
Similar to the first stage, the second stage of development – angiogenesis – also appears to be regulated by hypoxia. There is still no continuity between the separate vascular structures and there is no circulation of systemic blood within them. Later, the vessels coalesce to form the primary vascular plexus. The complex network of the subepicardial primary plexus expands along the dorsal and ventral interventricular sulcus, the atrioventricular sulcus, and then along the base of the truncus arteriosus throughout the myocardium. The vessels sprouting from the primary plexus form new septa and pillars within the vascular lumen, giving rise to the peritruncal ring of capillaries.
Only at the late stages of development will the vascular network gain access to the aortic root (arteriogenesis, Fig. 22.2). The vessels from the peritruncal ring sprout preferentially into the aorta, establishing multiple connections to the left and right aortic sinuses and much fewer to the posterior (noncoronary) aortic sinus. The hemodynamic changes associated with this access to the aortic root appear to initiate the vascular maturation of the network and its aortic orifices. The migration of smooth muscle cells and pericytes from the epicardium and the aortic root to the primitive vascular structures and their coalescence with them are the next step of a vascular maturation. As a result, stable arteries are formed; they grow radially and the peritruncal ring with its numerous vessels undergoes partial regression (Fig. 22.2). Defects at any stage of this complex development can lead to coronary anomalies.
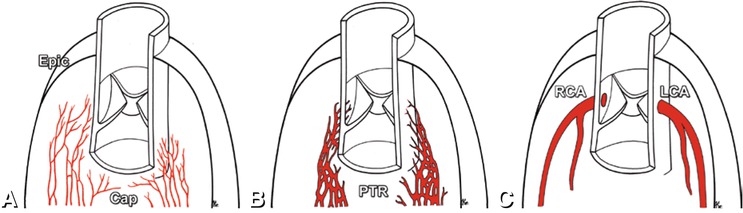

Fig. 22.2
Arteriogenesis of the coronary arteries. Multiple capillaries (Cap) form the primary vascular plexus beneath the epicardium (Epic in Panel a). Around the aorta and pulmonary trunk the capillaries coalesce to form a peritruncal ring (PTR in Panel b). Subsequently, multiple vessels extend towards the aorta and invade it. The peritruncal ring is reduced to the mainline coronary artery pattern with the right (RCA) and left coronary artery (LCA) attached to the aorta at the corresponding coronary sinuses (Panel c) (Modified from Bernanke et al. Anat Rec 2002)
22.2 Classification
Many different classifications of coronary artery anomalies have been proposed to categorize the enormous number of variations in coronary artery anatomy. This section gives an overview to serve as a basis for an adequate description of coronary anomalies and to facilitate identification of potentially clinically important variants. Most classifications of coronary anomalies rely exclusively on the description of the anatomic variant. The approach favored here is based on the classification proposed by Paolo Angelini (Circulation 2007). Other classifications group anomalous coronary arteries according to their origin, course, or termination. Coronary artery anomalies can also be functionally divided into hemodynamically significant (malignant type) and hemodynamically nonsignificant (benign type). The malignant type consists of coronary artery variants that can cause ischemia and even sudden cardiac death.
According to Angelini, coronary anomalies can be anatomically divided into anomalies of origin and course, of intrinsic anatomy, of termination, and anomalous anastomotic vessels (Table 22.1). The origin and course of the anomalous coronary artery determine its possible clinical impact to the greatest extent. Instead of an origin from the proper sinus of Valsalva, coronary arteries can arise from the opposite sinus and take different pathways to reach the myocardial area they are supplying. On its course to the opposite site, the coronary artery can pass anterior to the pulmonary artery (Fig. 22.3), posterior to the aorta, or interarterially between the aorta and pulmonary trunk, or even intraseptally (subpulmonic course). Coronary anomalies also occur in association with congenital heart disease, for example with tetralogy of Fallot, transposition of the great arteries or pulmonary atresia (Chap. 23).
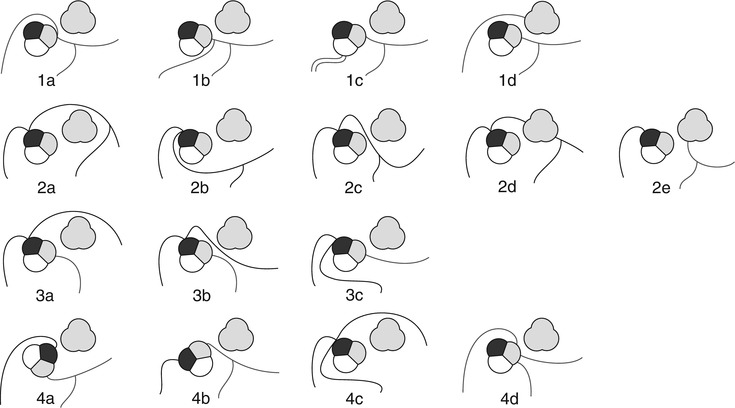
Table 22.1
Modified classification of coronary anomalies according to Paolo Angelini
A. Anomalies of origin and course |
1. Absent LM with split origin of LCA (Fig. 22.4). |
2. Anomalous location (high, low, commissural) of coronary ostium within aortic root or near proper aortic sinus of Valsalva |
3. Anomalous location of coronary ostium outside normal “coronary” aortic sinuses |
Right posterior aortic sinus, ascending aorta or aortic arch, left or right ventricle, pulmonary artery, etc. |
5. Single coronary artery (Fig. 22.22) |
B. Anomalies of intrinsic coronary arterial anatomy |
C. Anomalies of coronary termination |
1. Inadequate arteriolar/capillary ramifications |
D. Anomalous anastomotic vessels |

Fig. 22.3
Different courses of anomalously located coronary ostia at improper sinus with 1 showing anomalies of the right coronary artery (RCA) (a: anterior interarterial course, b: posterior course from left sinus of Valsalva, c: posterior course from the noncoronary sinus, d: anterior course from the pulmonary artery), 2 of the left main coronary artery (LM) (a: anterior course, b: posterior course, c: interarterial course, d: septal course, e: from the pulmonary artery (Bland–White–Garland syndrome)), 3 of the central branches of the left coronary artery (a: anterior course, b: interarterial course, c: posterior course), and 4 of both the right and left coronary arteries (a: orthotopic origins from clockwise rotated aortic bulb, b: orthotopic origins from counter-clockwise rotated aortic bulb, c: three origins from the right sinus, d: three origins from the left sinus). Aortic root: black: right sinus, grey: left sinus, white: noncoronary sinus. The pulmonary artery is shown completely in grey anterolateral to the aortic root (With permission from Schmitt et al. European Radiology 2005)
22.3 Clinical Relevance
The prevalence of coronary anomalies is reported to be 0.3 up to 5.6%, depending on the literature source. In a large study of over 100,000 patients examined by conventional coronary angiography the prevalence of coronary anomalies was found to be around 1.3%. There is also evidence that prevalence varies according to geographic region.
22.3.1 Benign Coronary Anomalies
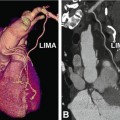
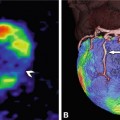
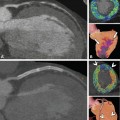
Coronary anomalies without compromise of blood flow to the myocardium or severe fistulas are considered benign. The simplest coronary anomaly is the absence of the left main coronary artery (split left coronary) with a separate origin of the left anterior descending and left circumflex coronary artery (Fig. 22.4). The most common course of a benign anomalous coronary artery is the prepulmonary (Fig. 22.5) and retroaortic path (Figs. 22.6, 22.7 and 22.8). Separate origins of diagonal or marginal branches from the aorta are also benign unless their course is compromised (Fig. 22.9)
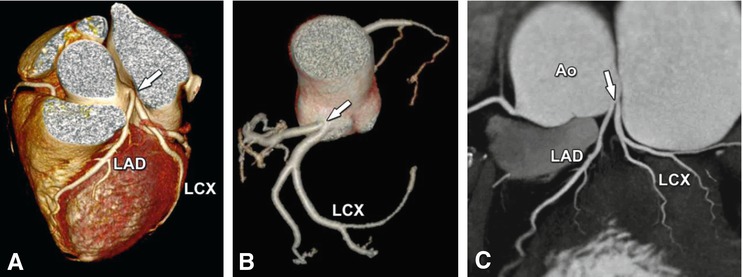
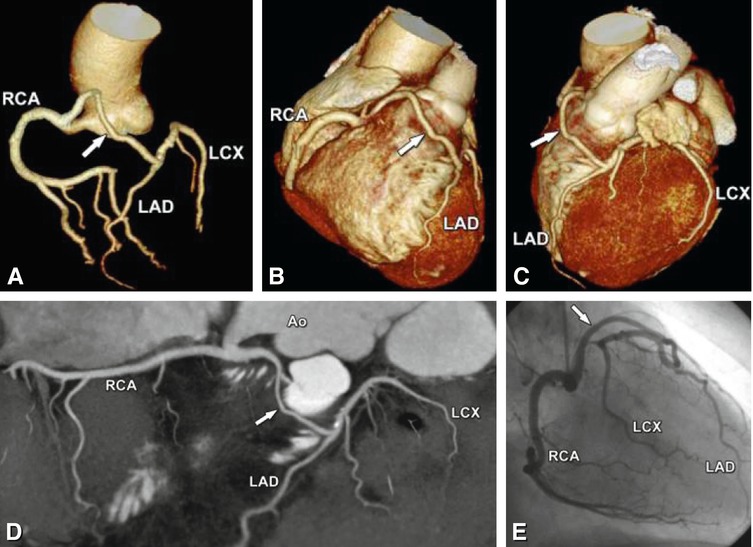
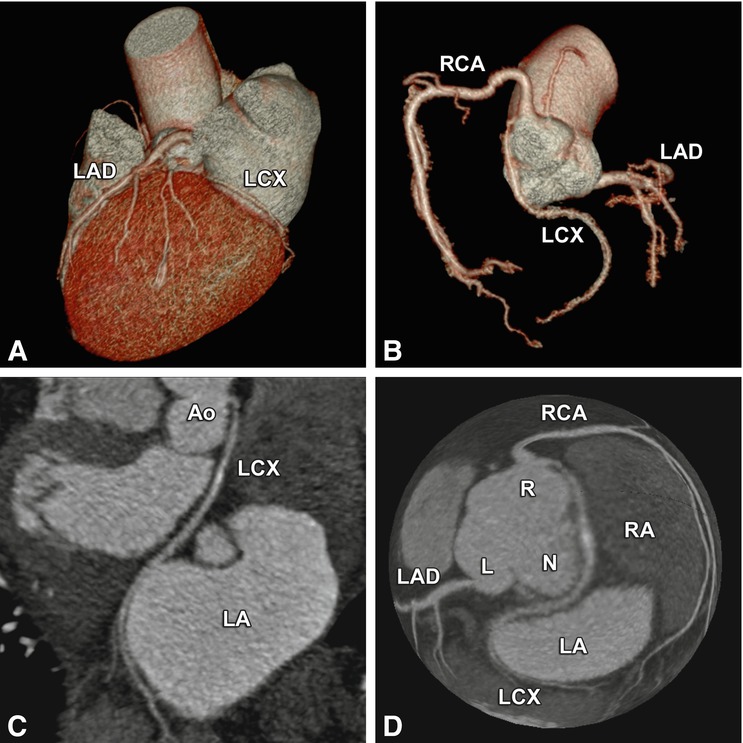
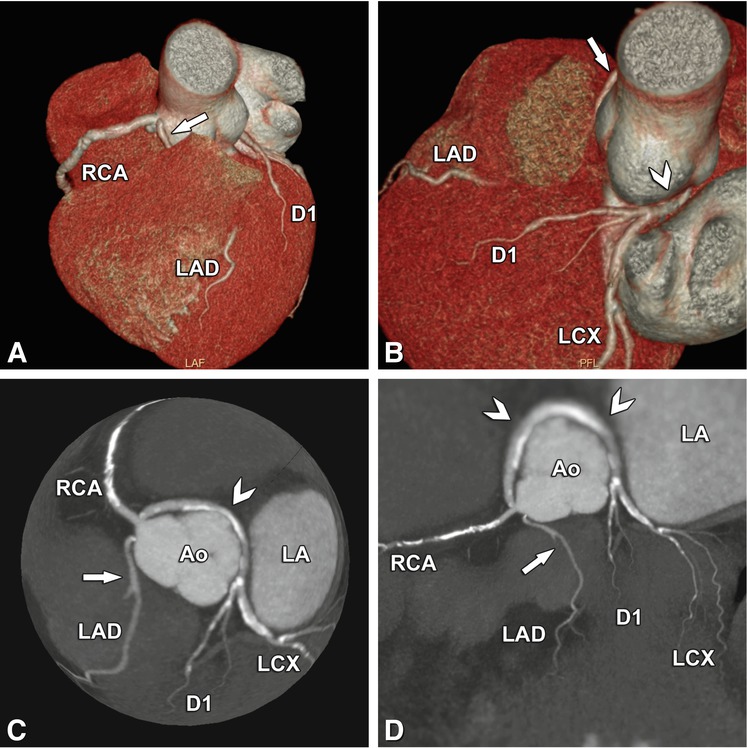





Fig. 22.4
Absence of the left main coronary artery (so-called split left coronary artery). The left anterior descending coronary artery (LAD) and the left circumflex coronary artery (LCX) arise separately (arrow) from the left sinus of Valsalva of the aorta (Ao) in a 68-year-old woman who was referred to cardiac CT to exclude coronary artery disease based on atypical chest pain. Panel a shows a cranial view of a three-dimensional volume-rendered image. The separate ostia cannot be recognized definitely. Better evaluation is possible on a volume-rendered reconstruction of the coronary tree (Panel b) or a two-dimensional map view (Panel c)

Fig. 22.5
Prepulmonary benign course of the left coronary artery arising from the right coronary artery after a short common trunk, which originates from the right sinus of Valsalva. The anomalously coursing left coronary artery (arrow in Panels a–d) passes anterior to the pulmonary artery to the anterior interventricular sulcus, where it splits into left anterior descending coronary artery (LAD) and left circumflex coronary artery (LCX). Due to the prepulmonary course of the left coronary artery, this anomaly is considered benign. The CT of this 52-year-old male patient was performed to exclude coronary artery disease based on hypertension and atypical chest pain. Conventional coronary angiography also showed the coronary anomaly, but the course of the proximal part of the left coronary artery was unclear (Panel e), therefore, the patient was referred for CT. The patient was medically treated and the further clinical course was uneventful. Panel a shows a volume-rendered reconstruction of the coronary tree. Panels b and c are three-dimensional volume-rendered images from two different views showing the anterior course of the anomalous left coronary artery, while Panel d gives an overview on a two-dimensional map view. Panel e shows the corresponding invasive angiography. Ao aorta

Fig. 22.6
Benign coronary anomaly with a retroaortic course of the left circumflex artery (LCX) originating from the right sinus of Valsalva (R) next to the ostium of the right coronary artery (RCA) in a 72-year-old female patient. CT was performed to exclude coronary artery disease because of chest pain and an abnormality of repolarization on exercise electrocardiography. Panels a and b show three-dimensional volume renderings of the heart and the coronary tree. The LCX passes retroaortically between the aorta and the right atrium (RA) and left atrium (LA) to reach the left atrioventricular sulcus as seen on a curved multiplanar reformation of the LCX and a globe view of the coronary tree, respectively (Panels c and d). Relevant coronary disease could be excluded and a minor artifact was seen in the proximal LCX. RCA right coronary artery, N noncoronary sinus, L left sinus of Valsalva, Ao aorta

Fig. 22.7
Another complex benign anomaly. The left anterior descending coronary artery (LAD) arises from a separate ostium next to the right coronary artery (RCA) and takes an intramuscular septal path before it appears in the interventricular sulcus (arrow, Panels a–d). The retroaortic path of the left circumflex coronary artery (LCX) and the first diagonal branch (D1, arrowheads, Panels b–d) can also be considered benign. Panels a and b are three-dimensional volume renderings from two different viewing angles, Panel c is a globe view, and Panel d is a two-dimensional map view, both showing the anomalous course nicely. Ao aorta, LA left atrium
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree