(T) Primary Tumor for Uterine | Adapted from 7th edition AJCC Staging Forms. | ||
TNM | FIGO | Definitions | |
TX | Primary tumor cannot be assessed | ||
T0 | No evidence of primary tumor | ||
Tis1 | Carcinoma in situ (preinvasive carcinoma) | ||
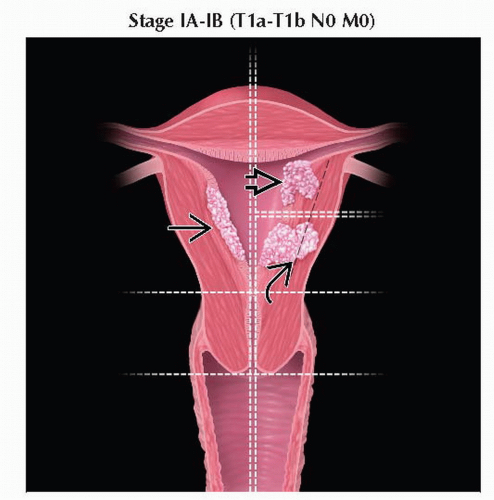
T1 | I | Tumor confined to corpus uteri | |
T1a | IA | Tumor limited to endometrium or invades < 1/2 of the myometrium | |
T1b | IB | Tumor invades ≥ 1/2 of the myometrium | |
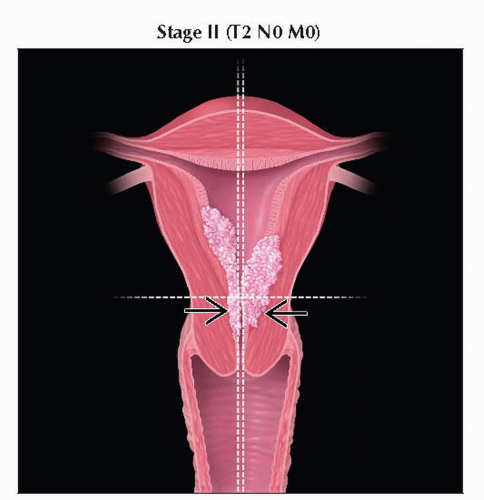
T2 | II | Tumor invades stromal connective tissue of the cervix but does not extend beyond | |
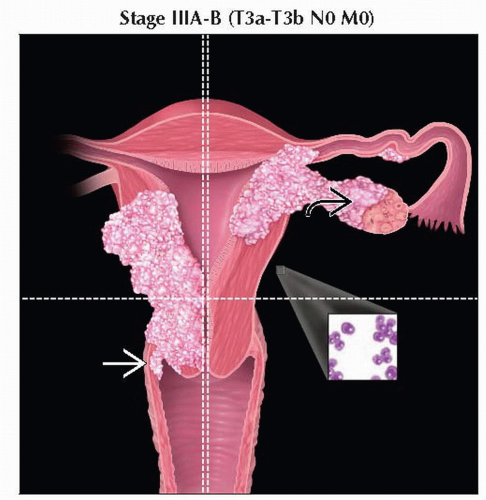
T3a | IIIA | Tumor invades serosa &/or adnexa (direct extension or metastasis) | |
T3b | IIIB | Vaginal involvement (direct extension or metastasis) or parametrial involvement | |
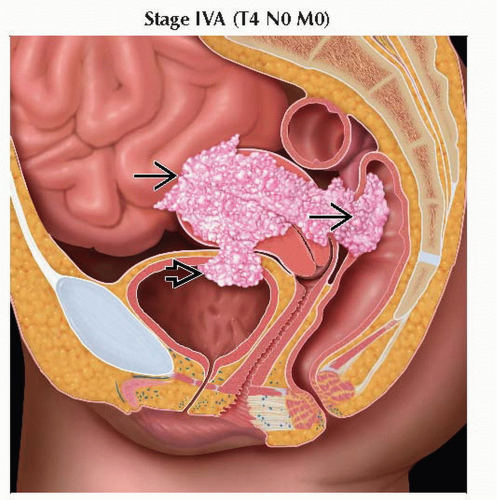
T4 | IVA | Tumor invades bladder mucosa &/or bowel mucosa (bullous edema is not sufficient to | |
1 FIGO no longer includes stage 0 (Tis). | |||
(N) Regional Lymph Nodes for | Adapted from 7th edition AJCC Staging Forms. | |
TNM | FIGO | Definitions |
NX | Regional lymph nodes cannot be assessed | |
N0 | No regional lymph node metastasis | |
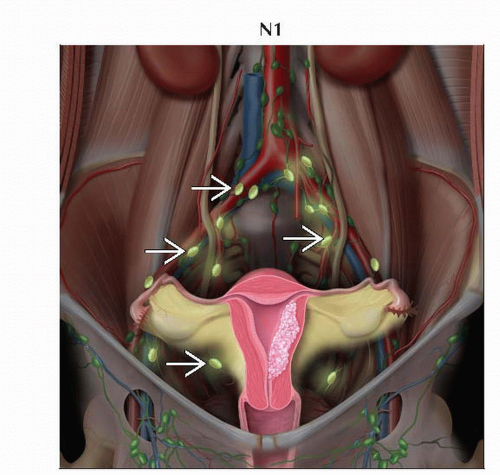
N1 | IIIC1 | Regional lymph node metastasis to pelvic lymph nodes |
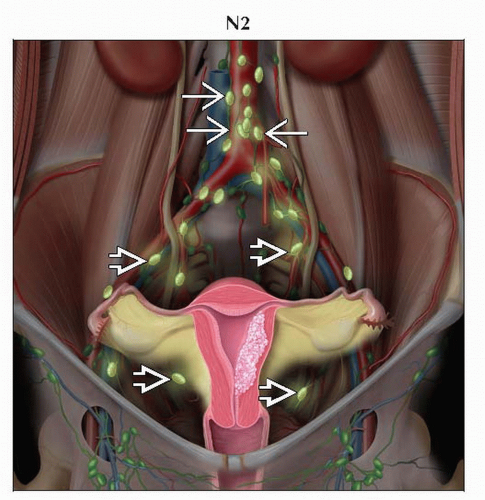
N2 | IIIC2 | Regional lymph node metastasis to paraaortic lymph nodes, with or without positive pelvic lymph nodes |
(M) Distant Metastasis for Uterine | Adapted from 7th edition AJCC Staging Forms. | |
TNM | FIGO | Definitions |
M0 | No distant metastasis | |
M1 | IVB | Distant metastasis (includes metastasis to inguinal lymph nodes intraperitoneal disease, |
AJCC Stages/Prognostic Groups for Uterine Carcinomas1 | Adapted from 7th edition AJCC Staging Forms. | |||
Stage | T | N | M | |
0 | Tis | N0 | M0 | |
I | T1 | N0 | M0 | |
IA | T1a | N0 | M0 | |
IB | T1b | N0 | M0 | |
II | T2 | N0 | M0 | |
III | T3 | N0 | M0 | |
IIIA | T3a | N0 | M0 | |
IIIB | T3b | N0 | M0 | |
IIIC1 | T1-T3 | N1 | M0 | |
IIIC2 | T1-T3 | N2 | M0 | |
IVA | T4 | Any N | M0 | |
IVB | Any T | Any N | M1 | |
1 Carcinosarcomas should be staged as carcinoma. | ||||
(T) Primary Tumor for Leiomyosarcoma and Endometrial | Adapted from 7th edition AJCC Staging Forms. | ||
TNM | FIGO | Definitions | |
TX | Primary tumor cannot be assessed | ||
T0 | No evidence of primary tumor | ||
T1 | I | Tumor limited to the uterus | |
T1a | IA | Tumor ≤ 5 cm in greatest dimensions | |
T1b | IB | Tumor > 5 cm | |
T2 | II | Tumor extends beyond the uterus, within the pelvis | |
T2a | IIA | Tumor involves adnexa | |
T2b | IIB | Tumor involves other pelvic tissues | |
T3 | III2 | Tumor infiltrates abdominal tissues | |
T3a | IIIA | 1 site | |
T3b | IIB | > 1 site | |
T4 | IVA | Tumor invades bladder or rectum | |
1 Simultaneous tumors of the uterine corpus and ovary/pelvis in association with ovarian/pelvic endometriosis should be classified as independent primary tumors. | |||
(N) Regional Lymph Nodes for | Adapted from 7th edition AJCC Staging Forms. | |
TNM | FIGO | Definitions |
NX | Regional lymph nodes cannot be assessed | |
N0 | No regional lymph node metastasis | |
N1 | IIIC | Regional lymph node metastasis |
(M) Distant Metastasis for | Adapted from 7th edition AJCC Staging Forms. | |
TNM | FIGO | Definitions |
M0 | No distant metastasis | |
M1 | IVB | Distant metastasis (excluding adnexa, pelvic and abdominal tissues) |
(T) Primary Tumor for | Adapted from 7th edition AJCC Staging Forms. | ||
TNM | FIGO | Descriptions | |
TX | Primary tumor cannot be assessed | ||
T0 | No evidence of primary tumor | ||
T1 | I | Tumor limited to the uterus | |
T1a | IA | Tumor limited to the endometrium/endocervix | |
T1b | IB | Tumor invades to < 1/2 of the myometrium | |
T1c | IC | Tumor invades ≥ 1/2 of the myometrium | |
T2 | II | Tumor extends beyond the uterus, within the pelvis | |
T2a | IIA | Tumor involves adnexa | |
T2b | IIB | Tumor involves other pelvic tissues | |
T3 | III2 | Tumor involves abdominal tissues | |
T3a | IIIA | 1 site | |
T3b | IIIB | > 1 site | |
T4 | IVA | Tumor invades bladder or rectum | |
1 Simultaneous tumors of the uterine corpus and ovary/pelvis in association with ovarian/pelvic endometriosis should be classified as independent primary tumors. | |||
(N) Regional Lymph Nodes for | Adapted from 7th edition AJCC Staging Forms. | |
TNM | FIGO | Descriptions |
NX | Regional lymph nodes cannot be assessed | |
N0 | No regional lymph node metastasis | |
N1 | IIIC | Regional lymph node metastasis |
(M) Distant Metastasis for Adenosarcoma | Adapted from 7th edition AJCC Staging Forms. | |
TNM | FIGO | Definitions |
M0 | No distant metastasis | |
M1 | IVB | Distant metastasis (excluding adnexa, pelvic and abdominal tissues) |
AJCC Stages/Prognostic Groups for | Adapted from 7th edition AJCC Staging Forms. | |||
Stage | T | N | M | |
I | T1 | N0 | M0 | |
IA1 | T1a | N0 | M0 | |
IB1 | T1b | N0 | M0 | |
IC2 | T1c | N0 | M0 | |
II | T2 | N0 | M0 | |
IIIA | T3a | N0 | M0 | |
IIIB | T3b | N0 | M0 | |
IIIC | T1, T2, T3 | N1 | M0 | |
IVA | T4 | Any N | M0 | |
IVB | Any T | Any N | M1 | |
1 Stage IA and IB differ from those applied for leiomyosarcoma and endometrial stromal sarcoma. | ||||
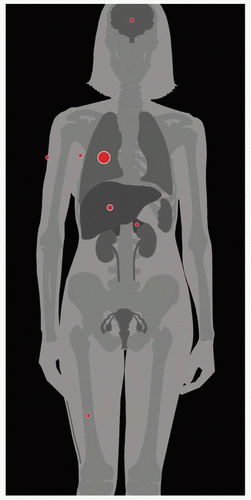
| METASTASES, ORGAN FREQUENCY | |
Lung | 32% | |
Liver | 7% | |
Other sites (adrenals, | 4% | |
Corpus uteri carcinoma is the most common gynecologic cancer in USA
Also most common gynecologic cancer in many other developed countries
95% of uterine malignancies are endometrial carcinomas
Malignancies of uterine corpus
Cancers above level of the cervical os involving upper 2/3 of uterus
Endometrial cancer can be divided into 2 types
Type I
Endometrioid histology
Includes the very common endometrioid adenocarcinoma
Makes up to 70-80% of new diagnoses in USA
Association with chronic estrogen exposure
Premalignant disease, such as endometrial hyperplasia, often precedes cancer
Type II
Nonendometrioid histology
Includes papillary serous and clear cell carcinomas
Aggressive clinical course
No association with estrogen exposure has been identified
Not associated with readily observable premalignant disease
Primary malignant tumors (WHO classification)
Endometrial carcinoma
Endometrioid adenocarcinoma
Several forms
Mucinous adenocarcinoma
Serous adenocarcinoma
Clear cell adenocarcinoma
Mixed cell adenocarcinoma
Squamous cell carcinoma
Transitional cell carcinoma
Small cell carcinoma
Others
Mesenchymal tumors
Endometrial stromal and related tumors
Endometrial stromal sarcoma, low grade
Endometrial stromal nodule
Undifferentiated endometrial sarcoma
Smooth muscle tumors
Leiomyosarcoma (epithelioid and myxoid variants)
Smooth muscle tumor of uncertain malignant potential
Leiomyoma, not otherwise specified
Miscellaneous mesenchymal tumors
Mixed epithelial and mesenchymal tumors
Carcinosarcoma
Adenosarcoma
Carcinofibroma
Adenofibroma
Adenomyoma
Gestational trophoblastic disease
Trophoblastic neoplasms
Choriocarcinoma
Placental site trophoblastic tumor
Epithelioid trophoblastic tumor
Molar pregnancies
Hydatiform mole
Nonneoplastic
Nonmolar trophoblastic lesions
Miscellaneous tumors
Sex cord-like tumors
Neuroectodermal tumors
Melanotic paraganglioma
Tumors of germ cell type
Others
Lymphoid and hematopoietic tumors
Malignant lymphoma
Leukemia
Direct extension
Most common
Lymphatic spread
Common nodes include
Pelvic (N1)
Paraaortic (N2)
Inguinal nodes (less common)
Hematogenous spread
Lungs
Liver
Bone
Skin
Brain (uncommon)
Peritoneal spread
Intraperitoneal implants
Common in papillary serous carcinoma
Comments
Endometrioid adenocarcinoma
Represents 75-80% of endometrial cancers
Genetics
Rare hereditary form
Lynch II family cancer syndrome
Nonpolyposis colorectal cancer
Ovarian cancer
Endometrial cancer
Type I endometrial cancers
Microsatellite instability
KRAS mutations
PTEN mutations
DNA mismatch repair defects
Mutations in p53
Less frequent
Late occurrence in development (differing from type II cancers)
Type II endometrial cancers
Mutations in p53
Common mutation
Nondiploid karyotype
Her-2/neu (c-erB-2) overexpression
Etiology
Carcinoma that spontaneously arises from endometrium that is atrophic or inert
Epidemiology & cancer incidence
Estimated 2009 statistics in USA for endometrial cancer overall
42,160 new cases
7,780 deaths
Represents 6% of all cancers in women
Risk factors
Estrogen hormone replacement therapy
Increases risk 2-10x
Obesity
Increases risk 2-20x
Polycystic ovarian syndrome (PCOS)
Increases risk 3x
Chronic anovulation
Increases risk 3x
Tamoxifen
Increases risk 2-3x
Nulliparity
Increases risk 2-3x
Early menarche
Increases risk 2-3x
Late menopause
Increases risk 2-3x
Hypertension
Increases risk 2-3x
Diabetes
Increases risk 2-3x
Demographics
Age
Most common in 6th and 7th decades of life
Ethnicity
Common in Eastern Europe and USA
Uncommon in Asia
Associated diseases, abnormalities
Endometrial hyperplasia
Associated with 20-40%
H&E
Histological patterns can be broadly divided into type I and type II endometrial cancers
Endometrioid histology
Nonendometrioid histology
Histopathologic types
Endometrioid carcinomas
Most common endometrial cancer (75-80% of cases)
Most are well differentiated
Back-to-back glandular proliferation of endometrium lacking intervening stroma
Villoglandular adenocarcinoma
Many villous fronds
Delicate central fibrovascular cores of villi and simpler branching pattern differentiates it from papillary serous carcinoma
Adenocarcinoma with benign squamous elements, squamous metaplasia, or squamous differentiation (adenoacanthoma)
Adenosquamous carcinoma (mixed adenocarcinoma and squamous cell carcinoma)
Mucinous adenocarcinoma
Serous adenocarcinoma (papillary serous)
Bizarre nuclei
Scant cytoplasm
Nuclear stratification
Marked nuclear atypia
Complex papillary architecture
Psammoma bodies (seen in 30% of cases)
Aggressive nature
Often presents late
Clear cell carcinoma
Possible patterns include tubulocystic, papillary, or solid
Psammoma bodies may be present but not as commonly as in papillary serous tumors
Clear cell appearance due to glycogen
Myometrial invasion is common (80% of carcinomas)
Aggressive nature
Often presents late
Squamous cell carcinoma
Undifferentiated carcinoma
Malignant mixed mesodermal tumors
Key diagnostic clues
Endometrial mass resulting in uterine cavity expansion
Localized tumors
Polypoid masses superficially attached to the endometrium
Diffuse tumors
Extensive endometrial invasion
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree