Craniofacial Trauma
Robert M. Barr
Alisa D. Gean
Tuong H. Le
Head Trauma
Imaging Strategy
Skull Films. Skull fracture, with or without signs of neurological injury, is an independent risk factor for a neurosurgically relevant intracranial lesion (1). Therefore, in the setting of clinically occult head trauma, the diagnosis of skull fracture serves to alert the clinician to the possibility of an immediate or delayed neurologically relevant intracranial lesion. However, conventional radiography itself (film or digital) is not sensitive for detection of intracranial pathology (2,3,4) and should not be performed in lieu of a detailed clinical history and physical examination. Patients who are judged to be at low risk for intracranial injury on the basis of a careful history and physical examination should be observed, and patients at high risk should be imaged by CT. Skull films virtually never demonstrate significant findings in the low-risk group and are inadequate to characterize or exclude intracranial injury in the high-risk group. Further, the absence of skull fractures on conventional radiography does not exclude significant intracranial injury. In fact, in one large autopsy series of patients with fatal head injuries, only 75% had skull fractures (5). The decision to obtain a head CT in the setting of trauma must be based on clinical grounds.
CT. Imaging of acute head trauma is performed to detect treatable lesions before secondary neurologic damage occurs. Currently, this is best performed by CT for several reasons: it is quick, widely available, and highly accurate in the detection of acute intra- and extra-axial hemorrhage, as well as skull, temporal bone, facial, and orbital fractures. Monitoring equipment is easily accommodated. CT images must be reviewed using multiple windows. A narrow window width is used to evaluate the brain, a slightly wider window width is used to exaggerate contrast between extra-axial collections and the adjacent skull, and a very wide window is used to evaluate the skull itself (see Figs. 3.1, 3.6). Contiguous 5-mm sections through the brain provide sufficient detail and can be obtained with modern scanners in less than 15 minutes. Thinner sections are used to evaluate the orbits, facial skeleton, and skull base. Intravenous contrast media is not used in the acute setting because it may mimic or mask underlying hemorrhage.
When CT is performed in unconscious patients with severe head injury, it may be wise to include routine coverage of the craniocervical junction. A study by Link et al found that 18% of these patients had fractures of C-1, C-2, or the occipital condyles and that roughly half of all fractures were missed by plain radiographs (6).
MRI has traditionally been less desirable than CT in the acute setting because of the longer examination times, difficulty in managing life-support and other monitoring equipment, and inferior demonstration of bone detail. MR, however, has been shown to be comparable or superior to CT in the detection of acute epidural and subdural hematomas and nonhemorrhagic brain injury (7,8). MR is also more sensitive to brain stem injury and to acute, subacute and chronic hemorrhage, especially with fluid-attenuated inversion recovery (FLAIR), gradient-recalled-echo (GRE) T2*-weighted, and susceptibility-weighted imaging (9,10,11). Diffusion-weighted and diffusion tensor imaging have improved detection of both acute and chronic neuronal injury (12,13,14,15). In the majority of cases, MR is the modality of choice for patients with subacute and chronic head injury and is recommended for patients with acute head trauma when neurologic findings are unexplained by CT. MR is also more accurate in predicting long-term prognosis. With the development of parallel imaging, faster imaging sequences, advanced imaging methods such as MR spectroscopy, MR perfusion, and magnetization transfer imaging, improved monitoring equipment, and greater scanner availability, MR will continue to play an increasing role in the evaluation of acute head trauma.
Scalp Injury
When interpreting CT scans for head trauma, it is helpful to begin by examining the extracranial structures for evidence of
scalp injury or radiopaque foreign bodies. Scalp soft-tissue swelling is often the only reliable evidence of the site of impact. The subgaleal hematoma is the most common manifestation of scalp injury and can be recognized on CT or MR as focal soft-tissue swelling of the scalp located beneath the subcutaneous fibrofatty tissue and above the temporalis muscle and calvarium.
scalp injury or radiopaque foreign bodies. Scalp soft-tissue swelling is often the only reliable evidence of the site of impact. The subgaleal hematoma is the most common manifestation of scalp injury and can be recognized on CT or MR as focal soft-tissue swelling of the scalp located beneath the subcutaneous fibrofatty tissue and above the temporalis muscle and calvarium.
Skull Fractures
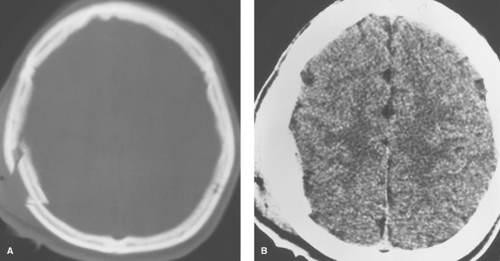
Nondisplaced linear fractures of the calvarium are the most common type of skull fracture. They may be difficult to detect on CT scans, especially when the fracture plane is parallel to the plane of section. Fortunately, isolated linear skull fractures do not require treatment. Surgical management is usually indicated for depressed and compound skull fractures, both of which are seen better on CT scans than on plain films (Fig. 3.1). Depressed fractures are frequently associated with an underlying contusion. Intracranial air (“pneumocephalus”) may be seen with compound skull fractures or fractures involving the paranasal sinuses. Thin-section CT using a bone algorithm is the best method to evaluate fractures in critical areas, such as the skull base, orbit, or facial bones. Thin sections can also be helpful to evaluate the degree of comminution and depression of bone fragments.
Temporal Bone Fractures
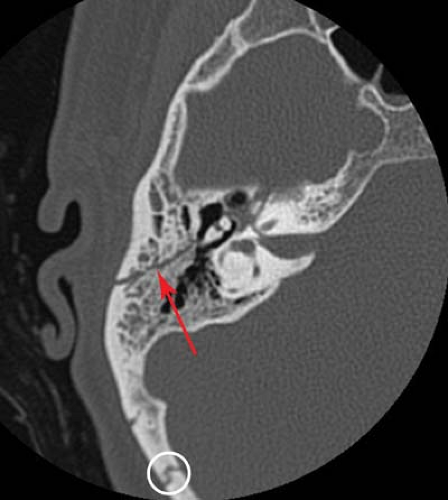
Thin-section, high-resolution CT scanning has led to a dramatic improvement in the ability to detect and characterize temporal bone fractures. Patients with fractures of the temporal bone may present with deafness, facial nerve palsies, vertigo, dizziness, or nystagmus. Clinical symptoms are often masked in the presence of other serious injuries. Physical signs of temporal bone fracture include hemotympanum, CSF otorrhea, and ecchymosis over the mastoid process (“Battle sign”). Temporal bone fractures may be first suspected on standard head CT scans performed to exclude intracranial injury. Findings such as opacification of the mastoid air cells, fluid in the middle ear cavity, pneumocephalus, or occasionally, pneumolabyrinth, should raise the suspicion of a temporal bone fracture. Optimal evaluation of a suspected temporal bone fracture requires thin-section (1 to 1.5 mm) axial and direct coronal CT imaging using a bone algorithm. With multidetector CT, thinner section axial imaging can be performed, and coronal reformats may be adequate for interpretation.
Fractures of the temporal bone can be classified either according to their orientation relative to the long axis of the petrous bone (16) or according to their involvement of the otic capsule (17,18). On the basis of the older Ulrich’s classification, if the fracture parallels the long axis of the petrous pyramid, it is termed a “longitudinal” fracture; fractures perpendicular to the long axis of the petrous bone are termed “transverse” fractures. “Mixed” fracture types also occur.
The longitudinal temporal bone fracture (Fig. 3.2) represents 70% to 90% of temporal bone fractures (19). It results from a blow to the side of the head. Complications include conductive hearing loss, dislocation or fracture of the ossicles, and CSF otorhinorrhea. Facial nerve palsy may occur, but it is often delayed and incomplete. Sensorineural hearing loss is uncommon.
The transverse temporal bone fracture usually results from a blow to the occiput or frontal region. Complications are usually more severe and include sensorineural hearing loss, severe vertigo, nystagmus, and perilymphatic fistula. Facial palsy is seen in 30% to 50% of these cases and is often complete (19). Transverse fractures may also involve the carotid canal or jugular foramen, causing injury to the carotid artery or jugular vein.
Mixed and oblique fracture types also occur, and the simple classification of fractures as longitudinal or transverse may not be sufficient (20). Otic capsule sparing fractures run anterolateral to the otic capsule, and are usually caused by direct blows to the temporoparietal region. With otic capsule violating fractures, the cochlea and the semicircular canals are damaged. These fractures are the results of direct impacts to the occipital region. Compared with otic sparing fractures, patients with otic capsule violating fractures are 2 to 5 times more likely to develop facial nerve injury, 4 to 8 times more likely to develop CSF leak, and 7 to 25 times more likely to experience hearing loss, as well as more likely to sustain
intracranial injuries such as epidural hematoma and subarachnoid hemorrhage (17,18).
intracranial injuries such as epidural hematoma and subarachnoid hemorrhage (17,18).
Head Injury Classification
Classification of Head Injury. Traumatic head injury can be divided into primary and secondary forms. Primary lesions are those that occur as a direct result of a blow to the head. Secondary lesions occur as a consequence of primary lesions, usually as a result of mass effect or vascular compromise. Secondary lesions are often preventable, whereas primary injuries, by definition, have already occurred by the time the patient arrives in the emergency department.
Primary lesions include epidural, subdural, subarachnoid, and intraventricular hemorrhage, as well as diffuse axonal injury (DAI), cortical contusions, intracerebral hematomas, and subcortical gray matter injury. Direct injury to the cerebral vasculature is another type of primary lesion.
Secondary lesions include cerebral swelling, brain herniation, hydrocephalus, ischemia or infarction, CSF leak, leptomeningeal cyst, and encephalomalacia.
Brain stem injury, which is also divided into primary and secondary forms, is discussed later in this chapter.
Primary Head Injury: Extra-Axial
Epidural hematomas are usually arterial in origin and often result from a skull fracture that disrupts the middle meningeal artery. The developing hematoma strips the dura from the inner table of the skull, forming an ovoid mass that displaces the adjacent brain. They may occur from stretching or tearing of meningeal arteries without an associated fracture, especially in children. Overall, skull fractures are seen in 85% to 95% of cases. In approximately a third of patients with an epidural hematoma, neurologic deterioration occurs after a lucid interval (21).
Most epidural hematomas are temporal or temporoparietal in location, though frontal and occipital hematomas can also occur. Venous epidural hematomas are less common and arterial epidurals and tend to occur at the vertex, posterior fossa, or anterior aspect of the middle cranial fossa. Venous epidural hematomas usually occur as a result of disrupted dural venous sinuses.
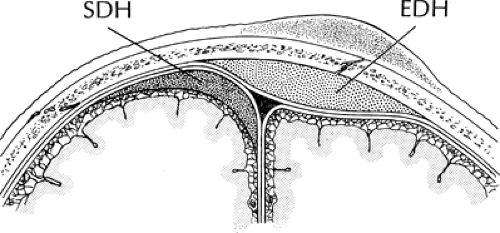
On CT, acute epidural hematomas appear as well-defined, high-attenuation lenticular or biconvex extra-axial collections (Fig. 3.3). Associated mass effect with sulcal effacement and midline shift is frequently seen. Bone windows usually demonstrate an overlying linear skull fracture. Because epidural hematomas exist in the potential space between the dura and inner table of the skull, they usually will not cross cranial sutures, where the periosteal layer of the dura is firmly attached (Fig. 3.4). Near the vertex, however, the periosteum forms the outer wall of the sagittal sinus and is less tightly adherent to the sagittal suture. Therefore, vertex epidurals, which are usually of venous origin from disruption of the sagittal sinus, can cross midline. Occasionally, an acute epidural hematoma will appear heterogeneous, containing irregular areas of lower attenuation. This finding may indicate active extravasation of fresh unclotted blood into the collection and warrants immediate surgical attention.
Subdural hematomas are typically venous in origin, resulting from stretching or tearing of cortical veins that traverse the subdural space en route to the dural sinuses. They may also result from disruption of penetrating branches of superficial cerebral arteries. Because the inner dural layer and arachnoid are not as firmly attached as the structures that make up the
epidural space, the subdural hematoma typically extends over a much larger area than the epidural hematoma. Patients with a subdural hematoma commonly present after acute deceleration injury from a motor vehicle accident or fall. The same mechanism can cause cortical contusions and DAI, which are frequently seen in association with acute subdural hematomas.
epidural space, the subdural hematoma typically extends over a much larger area than the epidural hematoma. Patients with a subdural hematoma commonly present after acute deceleration injury from a motor vehicle accident or fall. The same mechanism can cause cortical contusions and DAI, which are frequently seen in association with acute subdural hematomas.
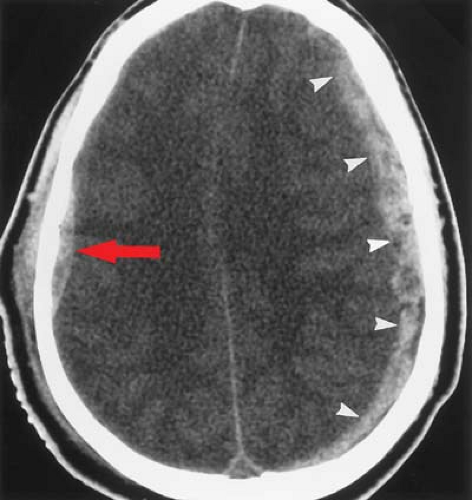
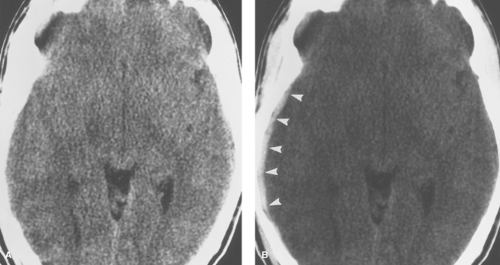
On axial CT, acute subdural hematomas appear as crescent-shaped extra-axial collections of high attenuation (Fig. 3.5). Small subdural hematomas may be masked by adjacent cortical bone when viewed on a narrow window width but will be seen with an intermediate window width (Fig. 3.6). Most subdural hematomas are supratentorial, located along the convexity. They are also frequently seen along the falx and tentorium. Because dural reflections form the falx cerebri and tentorium, subdural collections will not cross these structures (see Fig. 3.4). Unlike epidural hematomas, subdural hematomas can cross sutural margins and, in fact, are frequently seen layering along the entire hemispheric convexity from the anterior falx to the posterior falx. Diffuse swelling of the underlying hemisphere is common with subdural hematomas. Because of this, there may be more mass effect than would be
expected by the size of the collection and there may be little or no reduction in midline shift after evacuation of a hemispheric subdural hematoma.
expected by the size of the collection and there may be little or no reduction in midline shift after evacuation of a hemispheric subdural hematoma.
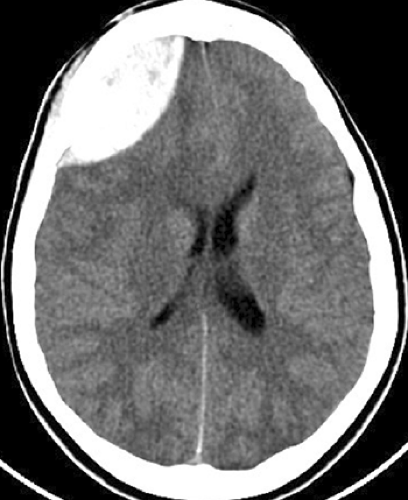
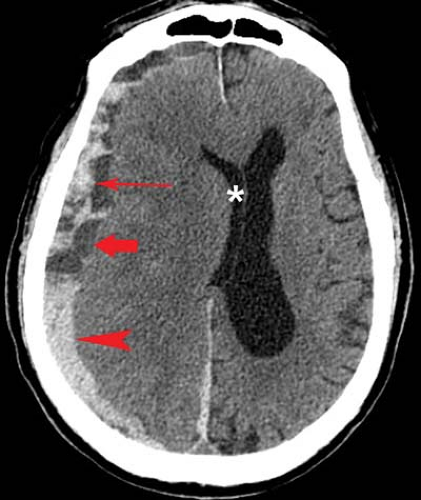
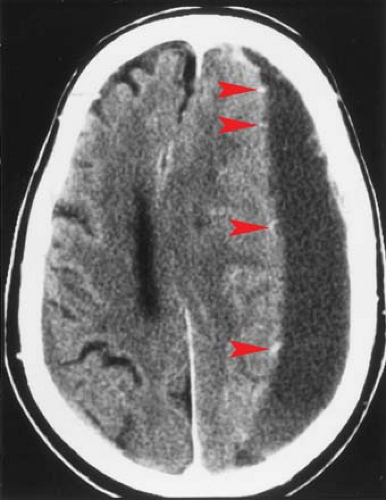
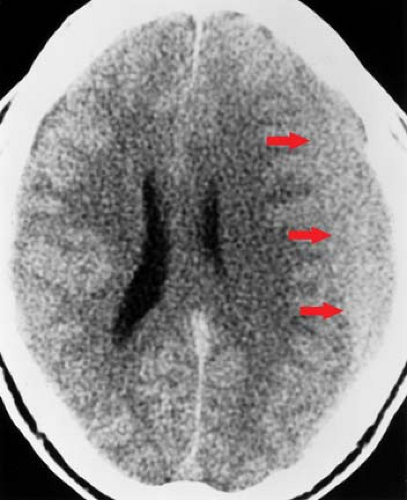
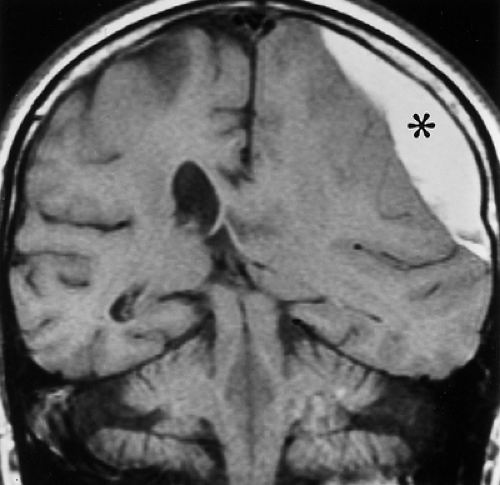
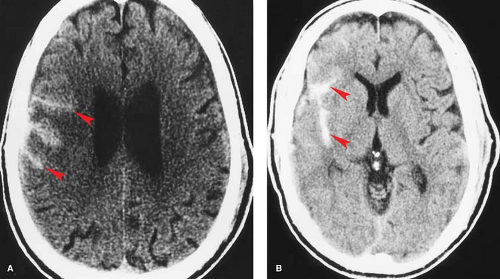
The CT appearance of subdural hematomas changes with time. The density of an acute subdural hematoma initially increases because of clot retraction. By the time most acute subdural hematomas are imaged, the collection is hyperdense, measuring 50 to 60 H, relative to normal brain, which measures 18 to 30 H. The density will then progressively decrease as protein degradation occurs within the hematoma. Occasionally, acute subdural blood may be isodense or hypodense in patients with severe anemia or active extravasation (“hyperacute” subdural hematoma). Rebleeding during evolution of a subdural hematoma causes a heterogeneous appearance from the mixture of fresh blood and partially liquefied hematoma (Fig. 3.7). A sediment level or “hematocrit effect” may be seen either from rebleeding or in patients with clotting disorders (Fig. 3.8). Chronic subdural hematomas have low attenuation values similar to CSF (Fig. 3.9). On noncontrast CT scans, it can be difficult to distinguish them from prominent subarachnoid space secondary to cerebral atrophy. Contrast enhancement can help by demonstrating an enhancing capsule or displaced cortical veins.
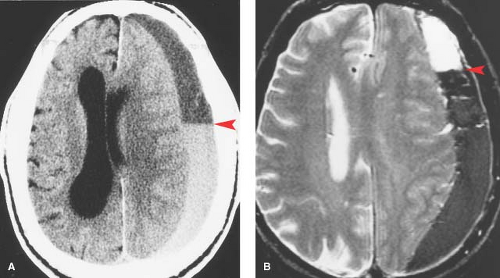
During the transition from acute to chronic subdural hematomas, an isodense phase occurs, usually between several days and 3 weeks after the acute event. Although the subdural hematoma itself is less conspicuous during this isodense phase, there are indirect signs on a noncontrast CT scan that should lead to the correct diagnosis. These include effacement of sulci, effacement or distortion of the white matter (“white matter buckling”), abnormal separation of the gray–white matter junction from the inner table of the skull (“thick gray matter mantle”), distortion of the ventricles, and midline shift (Fig. 3.10).
The MR appearance of subdural hematomas depends on the biochemical state of hemoglobin, which varies with the age of the hematoma. Acute subdural hematomas are isointense to
brain on T1WI and hypointense on T2WI. MR is particularly helpful during the subacute phase, when the subdural hematoma may be isodense or hypodense on CT scans. T1WI will demonstrate high signal intensity caused by the presence of methemoglobin in the subdural collection. This high signal clearly distinguishes subdural hematomas from most nonhemorrhagic fluid collections. MR also reveals that subacute subdural hematomas frequently have a lentiform or biconvex appearance when seen in the coronal plane (Fig. 3.11), rather than the crescent-shaped appearance that is characteristic on axial CT scans. The multiplanar capability of MR scanning is helpful in identifying small convexity and vertex hematomas that might not be detected on axial CT scans because of the similar attenuation of the adjacent bone.
brain on T1WI and hypointense on T2WI. MR is particularly helpful during the subacute phase, when the subdural hematoma may be isodense or hypodense on CT scans. T1WI will demonstrate high signal intensity caused by the presence of methemoglobin in the subdural collection. This high signal clearly distinguishes subdural hematomas from most nonhemorrhagic fluid collections. MR also reveals that subacute subdural hematomas frequently have a lentiform or biconvex appearance when seen in the coronal plane (Fig. 3.11), rather than the crescent-shaped appearance that is characteristic on axial CT scans. The multiplanar capability of MR scanning is helpful in identifying small convexity and vertex hematomas that might not be detected on axial CT scans because of the similar attenuation of the adjacent bone.
Subarachnoid hemorrhage is common in head injury but is rarely large enough to cause a significant mass effect. It results from the disruption of small subarachnoid vessels or direct extension into the subarachnoid space by a contusion or hematoma. On CT, subarachnoid hemorrhage appears as linear areas of high attenuation within the cisterns and sulci (Fig. 3.12). Subarachnoid collections along the convexity or tentorium can be differentiated from subdural hematomas by their extension into adjacent sulci. Occasionally, the only finding is apparent effacement of sulci when the sulci are filled with small amounts of blood. In patients who are found unconscious after an unwitnessed event, detection of subarachnoid hemorrhage may indicate a ruptured aneurysm, rather than trauma, as the primary cause. In such cases, contrast-enhanced CT angiography and/or conventional catheter angiography needs to be considered.
Hyperacute subarachnoid hemorrhage may be more difficult to detect on conventional MR than it is on CT scans because it can be isointense to brain parenchyma on T1W and
T2W images. However, FLAIR has been shown to be more sensitive than CT in detecting acute subarachnoid hemorrhage in animal model, especially when a high volume (1 to 2 mL) is present (10). Subacute subarachnoid hemorrhage may be better appreciated on MR because of its high signal intensity at a time when the blood is isointense to CSF on CT. Chronic hemorrhage on MR scans may show hemosiderin staining in the subarachnoid space, which appears as areas of markedly decreased signal intensity on T1- and T2-weighted sequences (“superficial hemosiderosis”). Subarachnoid hemorrhage may lead to subsequent hydrocephalus by impaired CSF resorption at the level of arachnoid villi.
T2W images. However, FLAIR has been shown to be more sensitive than CT in detecting acute subarachnoid hemorrhage in animal model, especially when a high volume (1 to 2 mL) is present (10). Subacute subarachnoid hemorrhage may be better appreciated on MR because of its high signal intensity at a time when the blood is isointense to CSF on CT. Chronic hemorrhage on MR scans may show hemosiderin staining in the subarachnoid space, which appears as areas of markedly decreased signal intensity on T1- and T2-weighted sequences (“superficial hemosiderosis”). Subarachnoid hemorrhage may lead to subsequent hydrocephalus by impaired CSF resorption at the level of arachnoid villi.
Intraventricular hemorrhage is commonly seen in patients with head injuries and can occur by several mechanisms. First, it can result from rotationally induced tearing of subependymal veins on the surface of the ventricles. Another mechanism is by direct extension of a parenchymal hematoma into the ventricular system. Third, intraventricular blood can result from retrograde flow of subarachnoid hemorrhage into the ventricular system through the fourth ventricular outflow foramina. Patients with intraventricular hemorrhage are at risk for subsequent hydrocephalus by obstruction either at the level of the aqueduct or arachnoid villi.
On CT, intraventricular hemorrhage appears as hyperdense material, layering dependently within the ventricular system (see Fig. 3.17B). Tiny collections of increased density layering in the occipital horns may be the only clue to intraventricular hemorrhage.
Primary Head Injury: Intra-Axial
Diffuse axonal injury (DAI) is one of the most common types of primary neuronal injury in patients with severe head trauma. As the name implies, DAI is characterized by widespread disruption of axons that occurs at the time of an acceleration or deceleration injury. The affected areas of the brain may be distant from the site of direct impact; in fact, direct impact is not necessary to cause this type of injury.
The incidence of DAI was likely underestimated until recently because of the difficulty in visualizing these lesions on existing imaging studies as well as on histologic specimens. DAI is much better seen by MR than CT. This factor accounts to a large degree for the increased success of MR at explaining neurologic deficits after trauma and in predicting long-term outcome. Though MR has improved the detection of DAI in patients suffering head trauma, the incidence of this form of injury is probably still underestimated. Newer imaging methods, such as diffusion-weighted and diffusion tensor imaging with three-dimensional tractography, have shown potential in improving the detection of white matter injury in both acute and chronic DAI (12,13,14,15).
Patients with DAI are most commonly injured in high-speed motor vehicle crashes. These lesions have not been seen as a consequence of simple falls, such as when a patient falls from the standing position. Loss of consciousness typically starts immediately after the injury and is more severe than in patients with cortical contusions or hematomas.
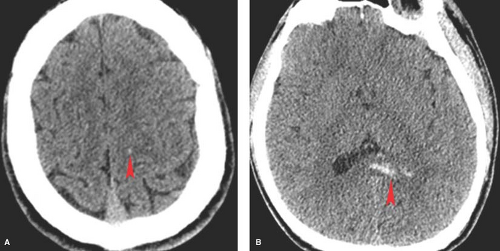
CT findings in DAI can be subtle or absent. Most common is the finding of small, petechial hemorrhages at the gray–white junction of the cerebral hemispheres or corpus callosum (Fig. 3.13). Ill-defined areas of decreased attenuation on CT may occasionally be seen with nonhemorrhagic lesions.
On MR, nonhemorrhagic DAI lesions appear as small foci of increased signal on T2WI (T2 prolongation) within the white matter (Fig. 3.14). The lesions tend to be multiple, with as many as 15 to 20 lesions seen in patients with severe head injury. If seen on T1WI, they appear as subtle areas of decreased intensity. Petechial hemorrhage causes a central hypointensity on T2WI and hyperintensity on T1WI within a few days as a result of intracellular methemoglobin. The conspicuity of DAI on MR diminishes over weeks to months as the damaged axons degenerate and the edema resolves. Residual findings might include nonspecific atrophy or hemosiderin
staining, which can persist for years and is especially obvious on gradient–echo sequences (Fig. 3.15).
staining, which can persist for years and is especially obvious on gradient–echo sequences (Fig. 3.15).
DAI is seen in characteristic locations that correlate with the severity of the trauma. Patients with the mildest forms of injury have lesions confined to the frontal and temporal white matter, near the gray–white junction. The lesions typically involve the parasagittal regions of the frontal lobes and periventricular regions of the temporal lobes. Patients with more severe trauma have DAI involving lobar white matter as well as the corpus callosum, especially the posterior body and splenium (Figs. 3.13 and 3.16). The corpus callosum accounts for approximately 20% of all DAI lesions (21). Initially thought to be caused by direct impact from the falx, experimental work shows that injury to the corpus callosum is most commonly caused by rotational shear forces, like all forms of DAI (22). The corpus callosum may be particularly susceptible to DAI because the falx prevents displacement of the cerebral hemispheres. DAI of the corpus callosum is almost always seen in association with lesions in the lobar white matter. DAI in the most severe cases involves the dorsolateral aspect of the midbrain and upper pons in addition to the lobar white matter and corpus callosum (see Brain Stem Injury).
Cortical contusions are areas of focal brain injury primarily involving superficial gray matter. Patients with cortical contusions are much less likely to have loss of consciousness at the time of injury than are patients with DAI. Contusions are
also associated with a better prognosis than DAI. They are very common in patients with severe head trauma and are usually well seen on CT scans. Contusions characteristically occur near bony protuberances of the skull and skull base. They tend to be multiple and bilateral and are more commonly hemorrhagic than DAI. Common sites are the temporal lobes above the petrous bone or posterior to the greater sphenoid wing, and the frontal lobes above the cribriform plate, planum sphenoidale, and lesser sphenoid wing (Fig. 3.17A). Less than 10% of lesions involve the cerebellum (23). Contusions can also occur at the margins of depressed skull fractures.
also associated with a better prognosis than DAI. They are very common in patients with severe head trauma and are usually well seen on CT scans. Contusions characteristically occur near bony protuberances of the skull and skull base. They tend to be multiple and bilateral and are more commonly hemorrhagic than DAI. Common sites are the temporal lobes above the petrous bone or posterior to the greater sphenoid wing, and the frontal lobes above the cribriform plate, planum sphenoidale, and lesser sphenoid wing (Fig. 3.17A). Less than 10% of lesions involve the cerebellum (23). Contusions can also occur at the margins of depressed skull fractures.
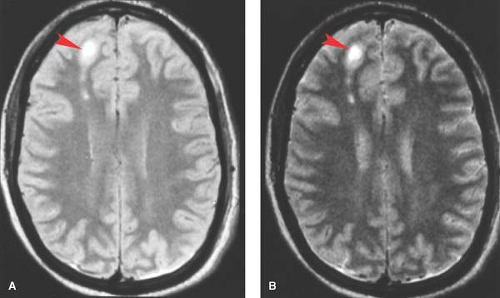 Figure 3.14. The MR Appearance of Acute Diffuse Axonal Injury. Proton-density (left) and T2-weighted (right) MR images show several adjacent foci of high signal (arrowheads) representing diffuse axonal injury in the right frontal parasagittal white matter. (Reprinted with permission from Gean AD. Imaging of Head Trauma. Philadelphia: Lippincott Williams & Wilkins, 1994:225.)
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|