Directed imaging is useful in assessing the thyroid gland. Nuclear scintigraphy reveals functional information about the thyroid gland, while cross-sectional imaging, including ultrasound, CT, and MR imaging provide important adjunctive anatomic information about the thyroid as well as about related structures in the neck, including the presence or absence of cervical and mediastinal lymphadenopathy, or extension of thyroid disease into adjacent soft tissues or the mediastinum. This article reviews the anatomy and physiology of the thyroid gland and addresses issues related to diseases affecting the thyroid gland, with an emphasis on neoplasms and the role of cross-sectional MR and CT imaging in the assessment of thyroid neoplasia.
The thyroid gland plays an important role in the regulation of many metabolic functions, including cardiac rate, lipid metabolism, skeletal growth, and heat production. As a result, the evaluation of patients with hypo- or hyperthyroidism requires an understanding of the hormonal functions that the thyroid gland performs. Thyroid nodules are detected by physical examination in up to 7% of the United States population. While the majority of nodules are benign, 7% to 10% represent thyroid carcinomas.
Directed imaging is useful in assessing the thyroid gland. Nuclear scintigraphy reveals functional information about the thyroid gland, while cross-sectional imaging, including ultrasound, CT, and MR imaging, provide important adjunctive anatomic information about the thyroid as well as about related structures in the neck, including the presence or absence of cervical and mediastinal lymphadenopathy, or extension of thyroid disease into adjacent soft tissues or the mediastinum.
This article reviews the anatomy and physiology of the thyroid gland and addresses Issues related to diseases affecting the thyroid gland, with an emphasis on neoplasms and the role of cross-sectional MR and CT imaging in the assessment of thyroid neoplasia.
Development and Anatomy of the Thyroid Gland
The thyroid gland develops in the first trimester of pregnancy, beginning around the fifth week of gestation, and is completed by the 10th gestational week. It develops from median and paired lateral anlages. The median anlage arises in the midline oropharynx at the fourth to fifth gestational week and gives rise to follicular thyroid tissue, which will secrete hormones. The lateral anlages are believed to arise from the ultimobranchial bodies, which are derived from the fourth and fifth branchial pharyngeal pouches at around the fifth week of gestation. They give rise to the parafollicular C-cells, which will secrete calcitonin and are thought to derive from the neural crest. By the 10th week in utero, the right and left lateral anlages fuse with the median anlage, resulting in the bilobed thyroid gland.
During fetal development, the thyroid gland descends from its place of origin, the foramen cecum at the base of the tongue, to its final adult destination in the lower neck. The thyroid is attached to the tongue base by the thyroglossal duct, which is lined by squamous epithelium. During the caudal descent of the gland, this duct elongates and subsequently degenerates. Abnormal development or aberrant caudal descent of the thyroid gland results in a spectrum of anomalies. Arrest of descent can occur anywhere from the tongue down to the lower neck. Failure of descent of the median thyroid anlage, or complete failure of descent of the thyroid, results in a lingual thyroid at the base of the tongue, the most common type of functioning ectopic thyroid tissue. In these cases, up to 75% of patients may have no functioning thyroid tissue in the neck. Overdescent of the thyroid may result in ectopic thyroid in the lower neck, in the mediastinum, or within mediastinal structures. On rare occasions, thyroid tissue may be found in the trachea, in the heart, or within ovarian teratomas (struma ovarii). Any pathology that may arise within normally located thyroid may also arise in ectopic tissue. Other developmental anomalies of the thyroid gland include agenesis or hemiagenesis (most often the left lobe) with normal formation of the contralateral lobe and isthmus.
The thyroid gland is shield-shaped and consists of right and left lobes adjoined by the isthmus, though occasionally the isthmus may be absent. The thyroid isthmus is anterior to the trachea (usually overlying the first through third tracheal rings). Infrequently, it may reside anterior to the cricoid cartilage. The thyroid is anterior to the prevertebral and paraspinal musculature, and deep to the sternothyroid and sternohyoid muscles. Usually, the thyroid gland terminates above the clavicles. However, substernal extension into the superior mediastinum may occur. An accessory lobe, the pyramidal lobe, may be present in 50% to 70% of people and usually arises from the isthmus and extends superiorly along the course of the distal thyroglossal duct. It may be attached to the hyoid bone. Uncommonly, the pyramidal lobe may arise from the medial right or left thyroid lobe. A pyramidal lobe is most commonly recognized in patients with Graves’ disease.
The visceral fascia, part of the middle layer of the deep cervical fascia, attaches the thyroid gland to the larynx and trachea. As a result, the gland or abnormalities related to it will move with the larynx during swallowing. The thyroid gland is encapsulated, and septae from the capsule extend into the substance of the gland.
The thyroid gland has a rich vascular supply with paired (right and left) superior and inferior thyroidal arteries. The superior thyroidal arteries are the first branches from the respective external carotid arteries, and travel inferiorly to the thyroid gland. The thyrocervical trunks originate from the subclavian arteries, and each gives rise to an inferior thyroidal artery. The thyroidea ima is an inconstant artery that arises directly from the aortic arch and helps supply the inferior thyroid gland. Superior and middle thyroidal veins drain into the internal jugular veins, and inferior veins drain into the innominate vein. The vagus nerve and the cervical sympathetic plexus innervate the thyroid gland. Sympathetic fibers descend from the sympathetic trunk, while parasympathetic fibers are along the course of the vagus nerve. This autonomic innervation is felt to strongly influence perfusion to the thyroid gland.
Endocrinology of the Thyroid Gland
The thyroid gland contains multiple lobules, each composed of multiple follicles. In these follicles, thyroglobulin is stored within colloid, and the follicular cells secrete hormones. Parafollicular (C) cells are dispersed throughout the stroma of the gland and secrete thyrocalcitonin. The primary function of the thyroid gland is the synthesis of hormones that regulate numerous metabolic functions. Two hormones, triiodothyronine (T3) and thyroxine (T4) are synthesized within the thyroid gland and released in response to a feedback mechanism with the pituitary-hypothalamic axis.
The synthesis of hormones within the thyroid is a regulated, systematic process. The first step involves trapping of iodide from the circulating plasma via active transport into the thyroid gland, where it is concentrated within follicular cells, and oxidized by thyroid peroxidase into its chemically active form. Subsequently, organification occurs. In this process, tyrosine residues on thyroglobulin molecules are iodinated to form monoiodotyrosine (MIT) and diiodotyrosine (DIT). The coupling of MIT and DIT forms T3, and the coupling of two molecules of DIT forms T4. T3 and T4 are released from thyroglobulin and secreted into the circulation in free and bound forms. Simultaneously, deiodination of free MIT and DIT occurs for iodide salvage and recycling within the thyroid gland. Aberrant organification usually results from enzymatic defects that interfere with the oxidation of iodide or the iodination of tyrosine. Iodide trapping may fail, but this is rare.
In the circulation, carrier proteins transport thyroid hormones. T4-binding globulin carries approximately 70% of T3 and T4, T4-binding preglobulin carries about 5% of T3 and 25% of T4, and albumin carries the remaining hormones. The active form of T3 and T4 is the free unbound form, representing only 0.3% of T3 and 0.03% of T4. T4 is synthesized entirely within the thyroid, while 80% of T3 is synthesized by peripheral conversion of T4 in the liver and muscle.
Several medications may temporarily interfere with the intrathyroidal transport or organification of iodide, including iodinated contrast materials frequently used in CT studies; oral cholecystographic agents; T4; liothyronine; antithyroid medications, such as propylthiouracil and methimazole; and amiodarone. These will alter radioactive iodine uptake in nuclear scintigraphy studies.
Endocrinology of the Thyroid Gland
The thyroid gland contains multiple lobules, each composed of multiple follicles. In these follicles, thyroglobulin is stored within colloid, and the follicular cells secrete hormones. Parafollicular (C) cells are dispersed throughout the stroma of the gland and secrete thyrocalcitonin. The primary function of the thyroid gland is the synthesis of hormones that regulate numerous metabolic functions. Two hormones, triiodothyronine (T3) and thyroxine (T4) are synthesized within the thyroid gland and released in response to a feedback mechanism with the pituitary-hypothalamic axis.
The synthesis of hormones within the thyroid is a regulated, systematic process. The first step involves trapping of iodide from the circulating plasma via active transport into the thyroid gland, where it is concentrated within follicular cells, and oxidized by thyroid peroxidase into its chemically active form. Subsequently, organification occurs. In this process, tyrosine residues on thyroglobulin molecules are iodinated to form monoiodotyrosine (MIT) and diiodotyrosine (DIT). The coupling of MIT and DIT forms T3, and the coupling of two molecules of DIT forms T4. T3 and T4 are released from thyroglobulin and secreted into the circulation in free and bound forms. Simultaneously, deiodination of free MIT and DIT occurs for iodide salvage and recycling within the thyroid gland. Aberrant organification usually results from enzymatic defects that interfere with the oxidation of iodide or the iodination of tyrosine. Iodide trapping may fail, but this is rare.
In the circulation, carrier proteins transport thyroid hormones. T4-binding globulin carries approximately 70% of T3 and T4, T4-binding preglobulin carries about 5% of T3 and 25% of T4, and albumin carries the remaining hormones. The active form of T3 and T4 is the free unbound form, representing only 0.3% of T3 and 0.03% of T4. T4 is synthesized entirely within the thyroid, while 80% of T3 is synthesized by peripheral conversion of T4 in the liver and muscle.
Several medications may temporarily interfere with the intrathyroidal transport or organification of iodide, including iodinated contrast materials frequently used in CT studies; oral cholecystographic agents; T4; liothyronine; antithyroid medications, such as propylthiouracil and methimazole; and amiodarone. These will alter radioactive iodine uptake in nuclear scintigraphy studies.
Clinical Manifestations of Thyroid Disease
Thyrotoxicosis is a clinical syndrome that develops when circulating levels of T4 and T3 are increased (thyrotropin is usually suppressed). Hyperthyroidism refers to sustained thyroid hyperfunction with increased thyroid hormone synthesis and release. Symptoms of thyrotoxicosis include warmth and flushing due to peripheral vasodilatation, heat loss, weight loss, myopathy, and increased appetite. Patients, especially children, may be hyperactive. Cardiac manifestations are more common in older patients and include tachycardia, palpitations, arrhythmias, and cardiomegaly.
Thyrotoxicosis associated with hyperthyroidism is most frequently seen with Graves’ disease, but may be seen with toxic multinodular goiter, and rarely a hyperfunctioning thyrotropin-secreting pituitary adenoma or thyroid neoplasm. Toxic multinodular goiter associated with hyperthyroidism (Plummer’s disease) commonly develops after 50 years of age and is related to a hyperfunctioning thyroid nodule. Thyrotoxicosis not associated with hyperthyroidism (low radioactive-iodine uptake) may be related to inflammatory thyroid disease or ectopic thyroid tissue (ovarian strumii), or it may be factitious (exogenous hormone use) ( Box 1 ).
Elevated thyroid function tests and normal 24-hour radioactive iodine uptake (10%–30%)
Plummer’s disease
Graves’ disease
Elevated thyroid function tests and low 24-hour radioactive iodine uptake (<10%)
Thyroiditis
De Quervain’s
Subacute lymphocytic
Struma ovarii
Factitious
Elevated thyroid function tests and high 24-hour radioactive iodine uptake (>30%)
Graves’ disease
Plummer’s disease
Thyroid ophthalmopathy, more common in women, is characterized by enlargement of the bellies of the extraocular muscles with sparing of the tendinous insertions, and is most commonly seen in Graves’ disease. The most common patterns of extraocular involvement are enlargement of all of the muscles, or of the inferior and medial muscle complexes. Isolated involvement of the lateral rectus muscle is rare and when present should raise suspicion for a different disease process, such as myositis or pseudotumor. Clinical signs and symptoms of thyroid ophthalmopathy include unilateral or bilateral proptosis due to increased orbital fat and an increase in the volume of the extraocular muscles related to edema and lymphocytic infiltrates, lid retraction that may result in corneal exposure, and decreased eye motion. Extraocular muscle enlargement may cause compression of the optic nerve at the orbital apex resulting in visual loss, which may require surgical decompression if refractory to medical therapy. Late in disease, contractures, fatty infiltration, and fibrosis of the extraocular muscles may lead to abnormal eye movements.
Hypothyroidism refers to decreased thyroid hormone synthesis with low T3 and T4 levels (serum thyrotropin is usually high). Primary hypothyroidism may be due to structural or functional abnormalities of the thyroid gland. In adults, this most often results from processes that destroy thyroid tissue, such as autoimmune disease or iodine 131 (I-131) treatment. In children, it may be related to enzyme deficiencies, defects in organification, or congenital anomalies, such as lingual thyroid or thyroid agenesis. Central hypothyroidism results from decreased thyroid stimulation by thyrotropin related to pituitary disease (secondary hypothyroidism) or hypothalamic thyrotropin-releasing hormone deficiency (tertiary hypothyroidism). Serum thyrotropin levels are normal in the presence of low serum T3 and T4 concentrations. Hypothyroidism occurring in the prenatal period or during infancy results in cretinism if not readily identified and treated. Hypothyroidism in older children and adults (myxedema) has variable clinical manifestations ranging from fatigue to coma, depending upon the degree and duration of hypothyroidism.
Secondary manifestations of thyroid disease are frequently responsible for clinical presentation. Marked enlargement of the thyroid gland most common in multinodular goiter, but also seen in neoplastic and inflammatory processes, may compress the adjacent esophagus, causing dysphasia, and trachea, causing respiratory distress. The recurrent laryngeal nerve travels in the tracheoesophageal groove, and thyroid lesions that affect this area may present with vocal cord paralysis. Cervical lymphadenopathy in the presence of a thyroid mass, or extension of a thyroid mass outside the capsule into adjacent structures, such as the trachea, is usually indicative of thyroid neoplasia.
Imaging the Thyroid Gland: the Radiologist’s Arsenal
Nuclear Scintigraphy
Nuclear scintigraphy provides excellent functional information about the thyroid gland because the radionuclides used to image the gland do so by using some step of hormone synthesis in the thyroid gland. The primary role of scintigraphy is in the evaluation of patients with tests that show abnormal thyroid function, especially hyperthyroidism. Scintigraphy determines if the cause of hyperthyroidism is a diffuse process, such as Graves’ disease, or an autonomously functioning nodule. Scintigraphy of a focal thyroid mass in a euthyroid patient may determine whether a lesion is functioning (very low incidence of malignancy) or nonfunctioning “cold,” a feature carrying a reported risk of malignancy ranging from 8% to 25%. However, 95% of thyroid nodules are cold and, hence, nuclear scintigraphy plays a minor role in the workup of dominant thyroid nodules.
Morphologic detail of the thyroid gland is obtained using technetium (Tc-99m) pertechnetate or iodine 123 (I-123). Tc-99m is trapped by the thyroid allowing an estimate of thyroid activity, whereas I-123 is also organified providing a true assessment of diffuse or focal regions of uptake. For routes of administration and doses of radionuclides see Table 1 . Imaging is performed approximately 20 minutes following administration of Tc-99m pertechnetate, 4 to 24 hours after oral ingestion of I-123, and 24 to 72 hours following administration of I-131. The normal thyroid gland shows homogenous radionuclide uptake. The isthmus may demonstrate slightly less activity than the thyroid lobes.
| Radionuclide | Administration | Dose | Half-life |
|---|---|---|---|
| Tc-99m | Intravenous | 74–370 MBq | 6.02 h |
| I-123 | Oral | 7.4–14.8 MBq | 13.6 h |
| I-131 (diagnostic) | Oral | 1.11–3.7 MBq | 8.05 d |
| I-131 (whole body) a | Oral | 74–185 MBq | — |
| I-123 (whole body) a | Oral | 37–92.5 MBq | — |
| I-131 (treatment) b | Oral | 3700 MBq | — |
| I-131 (treatment) c | Oral | 3700–7400 MBq | — |
a Diagnostic whole-body scan following thyroidectomy to evaluate for residual thyroid tissue, or to detect metastases; to detect ectopic thyroid tissue, such as struma ovarii, in hyperthyroid patients with no demonstrable iodine uptake in the thyroid.
b Cancer treatment following thyroidectomy to ablate residual thyroid tissue (may require hospital admission, depending on dose).
c Cancer treatment to ablate thyroid metastases (may require hospital admission, depending on dose).
I-123 is used for obtaining the 24-hour thyroid iodine uptake. Thyroid uptake reflects the percentage of the dose given to the patient that is accumulated within the gland, corrected for radioactive decay. Normal 24-hour uptake ranges from 10% to 30%. Several medications, iodine-containing topical solutions, and intravenous iodinated contrast agents used for imaging may temporarily interfere with the organification of iodide, altering radioactive iodine uptake measurements for as long as 6 weeks. The uptake of radioactive iodine may be reduced by as much as 50% 1 week following injection of iodinated contrast for a CT scan. In over one third of patients with underlying thyroid disease, temporary changes in thyroid function may occur following injection of iodinated contrast material. Therefore, if cross-sectional imaging is felt to be necessary in a patient who will also be studied with nuclear scans using iodinated radionuclides, MR imaging can be obtained. If the patient has a contraindication for MR imaging, then CT should be performed without intravenous contrast administration and, if contrast is desired, the CT should be performed following evaluation with nuclear scintigraphy.
Iodinated radionuclides may be used both in the imaging evaluation and treatment of patients with thyroid cancers that concentrate iodine. They are particularly useful in the follow-up of patients after thyroidectomy to evaluate for residual thyroid tissue in the operative bed, as well as to assess for recurrent or distant metastatic disease (see thyroid malignancies).
Fluorine-18-labeled fluorodeoxyglucose (FDG) positron emission tomography (PET) is playing an increasing role in the evaluation of select patients treated for thyroid cancer. It may be particularly useful in metastatic thyroid tumors that do not concentrate radioiodine. It is increasingly used in the workup of rising serum thyroglobulin levels in patients following thyroidectomy with clinically negative examinations, and is valuable in increasing physician confidence in the detection of disease as well as in the planning of management of recurrent disease. Whole-body scans are obtained to identify regions of FDG uptake. Potential pitfalls include indolent or well-differentiated thyroid tumors that take-up FDG poorly, and FDG uptake that may not be related to metastatic thyroid cancer.
Ultrasonography
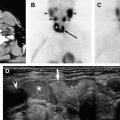
Sonography is the primary imaging modality for the evaluation of thyroid disease. Compared with other imaging modalities sonography provides the highest resolution and therefore is best able to detect and characterize diffuse and focal thyroid abnormalities. Ultrasound may also be used to guide fine needle aspiration of nodular disease within the thyroid ( Fig. 1 ), or to guide aspiration of suspicious cervical lymph nodes in the setting of thyroid cancer.
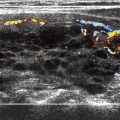
Real-time ultrasound is usually performed with a high-resolution linear array transducer ranging from 7.5 to 12 MHz. The patient is placed in a supine position and the neck is mildly hyperextended. The thyroid gland is imaged in its entirety both in transverse and longitudinal planes. The carotid arteries and jugular veins are posterior and lateral to the thyroid lobes, respectively, and provide excellent anatomic markers during the examination. The examination also includes assessment of the midline neck from the sternal notch to the hyoid bone to detect lesions, such as thyroglossal duct cysts. The normal thyroid gland is uniformly hyperechoic relative to the strap muscles and homogeneous in echotexture ( Fig. 2 ). The superior and inferior thyroidal arteries and veins and their intrathyroidal branches are often identified.
Limitations of sonography include the skill of the operator; the inability of sonography to assess the deep structures of the neck, such as the skull base; and limited detection of retrotracheal and intrathoracic extension of an enlarged thyroid due to acoustic impedance caused by air in the trachea or adjacent bones. Ultrasound is also limited in detecting extension of thyroid malignancy into the trachea, esophagus, or other adjacent soft tissue structures of the neck.
Cross-Sectional Imaging: CT and MR Imaging
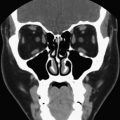
CT and MR imaging provide important adjunctive anatomic information in select clinical scenarios, especially in assessing advanced thyroid carcinomas at presentation, as well as in the evaluation of recurrent thyroid cancer following thyroidectomy. These modalities may play a critical role in the detection of lymph node metastases, especially nodal metastases in clinically occult areas that are poorly assessed by ultrasound (retropharynx and mediastinum), and are critical in evaluating extension of thyroid disease into adjacent tissues in the neck. Specifically, these modalities accommodate assessments of invasion of the adjacent musculature, esophagus, trachea/larynx, and jugular vein ( Fig. 3 ). The information provided by CT and MR imaging in these cases is invaluable in planning the surgical approach.
Because of its iodide content, the normal thyroid gland has a density of 80 to 100 Hounsfield units on CT. The intravenous injection of iodinated contrast material usually diffusely increases the density of the gland. Iodinated contrast material may provide additional information about thyroid lesions. However, because the contrast contains iodine, it will alter radioactive iodine uptake measurements for up to 6 weeks following the study. Therefore, in patients in whom nuclear scintigraphy is also going to be performed, contrast should not be administered. If both functional and cross-sectional studies are felt to be necessary, nuclear imaging can be performed before CT, or MR imaging may be used in conjunction with scintigraphy, as the contrast agent used in MR (gadolinium) does not interfere with iodide uptake or organification by the thyroid.
Imaging of the thyroid gland with MR should be multiplanar with multiple pulse sequences, including unenhanced sagittal and axial T1-weighted (T1-W) images, as well as axial fast spin-echo T2-weighted (T2-W) images with the application of fat saturation. Following the intravenous administration of gadolinium, axial T1-W images with the application of fat saturation are acquired. Because lesions may be hyperintense (bright) and fat in the neck is hyperintense on T1-W and T2-W images, fat-suppression helps to increase lesion conspicuity. On T1-W images, the normal thyroid gland shows homogeneous signal intensity slightly greater than that of the neck musculature. On T2-W images, the thyroid gland is hyperintense relative to the neck musculature. Following contrast administration, the normal gland enhances homogenously.
Nodular Diseases of the Thyroid Gland
Thyroid Goiter
Goiter refers to any enlargement of the thyroid gland. Nodular goiter is characterized by excessive growth with structural or functional transformation of one or several areas within an otherwise normal gland. In the absence of autoimmune thyroid disease, thyroiditis, thyroid dysfunction, and thyroid malignancy, this condition is termed simple nodular goiter. The pathogenesis of simple nodular goiter is related to genetic and environmental factors, most importantly iodine deficiency. To compensate for inadequate thyroid hormone output, follicular epithelium undergoes compensatory hypertrophy to achieve a euthyroid state. Hypo- or hyperthyroidism may develop. Initially, the goiterous enlargement is diffuse. However, with time it usually becomes nodular. If the impediment to thyroid hormone output abates, the thyroid gland may revert to normal during the diffuse state.
Diffuse nontoxic goiter represents diffuse, nonnodular enlargement of the thyroid associated with a euthyroid state. There are two stages. The first is hyperplasia (follicular cell growth) characterized by diffuse glandular enlargement and hyperemia. The second stage is colloid involution, which occurs when a euthyroid state is maintained. Endemic goiters are prevalent in iodine-deficient areas. There is a female predominance and a peak incidence at puberty. Most simple goiters progress to multinodular goiter that may remain nontoxic and are characterized by nodularity, focal hemorrhage, focal calcifications, and cyst formation. Glandular enlargement may be asymmetric, involving one lobe more than the other, or the isthmus. Thyroid goiters may extend substernally into the anterior mediastinum. A solitary cold nodule in a multinodular gland has similar cancer rates as those of a solitary cold nodule in a normal gland. Therefore, a dominant or enlarging mass within a goiterous thyroid raises concern for a malignancy and should be histologically sampled.
Sonography is often able to differentiate among the various causes of an enlarged thyroid gland. On ultrasound, CT, or MR imaging, multinodular goiter has multiple nodules of varying size. These nodules commonly contain complex cystic areas, representing colloid often mixed with areas of hemorrhage and necrosis. Dystrophic calcifications are common and typically are coarse and large. On T1-W MR images, foci of high signal intensity may represent cysts with colloid or hemorrhage. On T2-W MR images, diffuse heterogeneity may be present, and nodules as small as 3 to 5 mm can be visualized. Unlike ultrasound and CT, calcifications may be difficult to detect on MR imaging.
CT and MR imaging are the most valuable imaging modalities in assessing secondary manifestations of goiter, including compression and displacement of the trachea, esophagus, and adjacent vessels ( Fig. 4 ). Substernal and mediastinal extension are readily detected. When symptoms related to compression of the aerodigestive tract or vessels occur in elderly patients, nonsurgical candidates, or those refusing surgery, therapy with I-131 may be effective in reducing thyroid volume.