A. Nongenetic renal cysts
1. Simple cyst
2. Complex cyst
3. Multicystic dysplastic kidney
4. Cystic neoplasia
5. Medullary sponge kidney (MSK)
B. Genetic cystic renal diseases
1. Autosomal dominant polycystic kidney disease (ADPKD)
2. Autosomal recessive polycystic kidney disease (ARPKD)
3. Nephronophthisis-medullary cystic disease complex (NPH/MCD)
4. Glomerulocystic kidney disease (GCKD)
5. Cysts associated with multiorgan syndromes (e.g., tuberous sclerosis)
Nongenetic cysts include isolated or sporadic cysts that are mainly unilateral. On the other hand, genetic cystic diseases usually have extrarenal and multiorgan involvement and are bilateral as a rule. Besides revolutionary genetic progresses, high-resolution sonographic imaging has made a great impact in early detection of tiny renal cysts, thus facilitating early diagnosis. The increasing use of antenatal and perinatal sonography has also contributed to early incidental detection of renal cysts and cystic diseases.
Cysts are probably the most frequent abnormal findings to be described in kidney imaging reports. If they are few and small in an elderly patient, they may be ignored as a natural aging process because incidence of simple cysts increase with age, reaching above 50 % after the fifth decade. However, if multiple cysts are seen in a child or in a young to middle-aged person, they should be interpreted with caution. If additional findings such as small or large kidneys and parenchymal changes are present, and cysts are bilateral, then certain genetic cystic diseases should be suspected. Other organ involvements and family history may help confirm the diagnosis of a certain disease based on imaging findings. Nevertheless, some cases still need pathological confirmation, genetic testing, or follow-up for a final diagnosis.
Complex renal cysts constitute a small part of all cysts and cystic neoplasms. Multicystic dysplastic kidney, a congenital but nongenetic disease of kidneys, has an incidence of 1 per 1,000–4,000 live births. It is the second most common cause of an abdominal mass in a newborn. A more common cystic disease, medullary sponge kidney, which has an incidence of 0.5 %, is often asymptomatic.
The prototype and the most common genetic cystic kidney disease is autosomal dominant polycystic kidney disease (ADPKD) with a prevalence of 1/500. The recessive form (ARPKD) occurs approximately 1 in 20,000 births. Among the rest of the genetic cystic kidney diseases, juvenile nephronophthisis is the most common and affects 1 per 5,000 persons. Medullary cystic and glomerulocystic kidney diseases are much rarer. Tuberous sclerosis and von Hippel-Lindau disease are other rare but well-known diseases (less than 1 in 10,000 persons) that may be associated with renal cysts.
Imaging and Pathology
Ultrasonography is the basic imaging method preferred for both detection and description of renal cysts and cystic diseases. There are several reasons for this choice. Besides its well-known advantages for antenatal and pediatric age, the probable contraindications for use of contrast material in certain cystic diseases with renal insufficiency make it more suitable in most instances.
Nongenetic Renal Cysts
Simple Cyst
Simple Cortical Cyst
General Information
Simple cysts are believed to arise from obstructed collecting duct tubules.
They are usually 4 cm or less but may reach considerable size of more than 10 cm.
Although simple cysts are mainly located in the cortical region, they may be peripheral in location. In adults, simple cysts do not need to be monitored or treated unless they are too big to cause any pain or compression of renal tissue. Percutaneous US-guided sclerotherapy should be the first-line management method for these cysts.
Intracystic hemorrhage, infection, and rupture of simple renal cysts are indeed very rare complications and are probably overestimated in the literature.
Imaging
Plain Film Radiography
Plain films are not used any more in diagnosis and differential diagnosis of cysts. They can be detected on plain films if they are big enough to create a separate density.
Intravenous Pyelography (IVP)
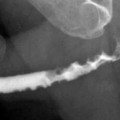
On IVP, compression caused by renal cysts on the collecting system can be appreciated but cystic-solid differentiation of a mass would not be possible (Fig. 3.1).
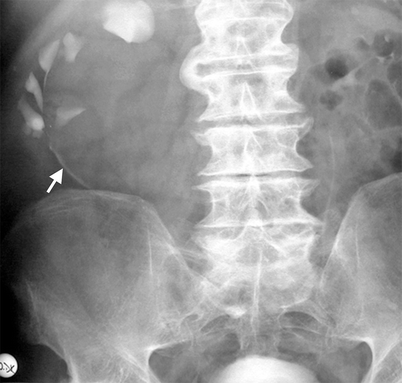
Fig. 3.1
Simple cyst. An elderly man with a large right renal cyst. IVP shows compression, distortion and dilatation of the collecting system. Linear opacity represents compressed calices by the cyst (arrow)
Ultrasonography
A simple cyst has three features:
Cyst with a thin wall or imperceptible wall
Anechoic center
And through transmission or posterior acoustic enhancement (Fig. 3.2)
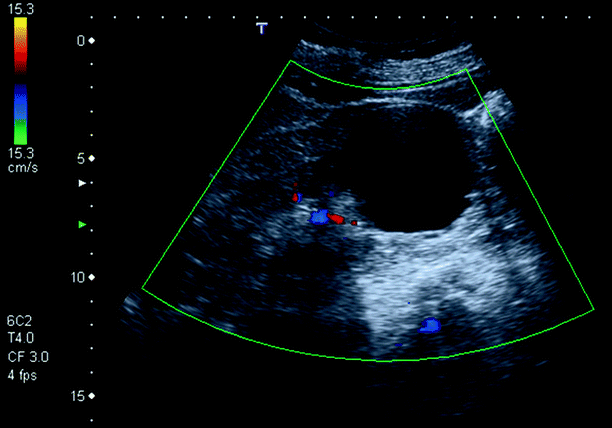
Fig. 3.2
Simple cyst. A 65-year-old woman with anechoic, thin-walled 5 cm cyst with posterior acoustic enhancement in the lower pole of her left kidney. Color Doppler does not demonstrate any blood flow
Acquired simple cysts are common in chronic kidney diseases, especially if the patient is on long-term dialysis (Fig. 3.3).
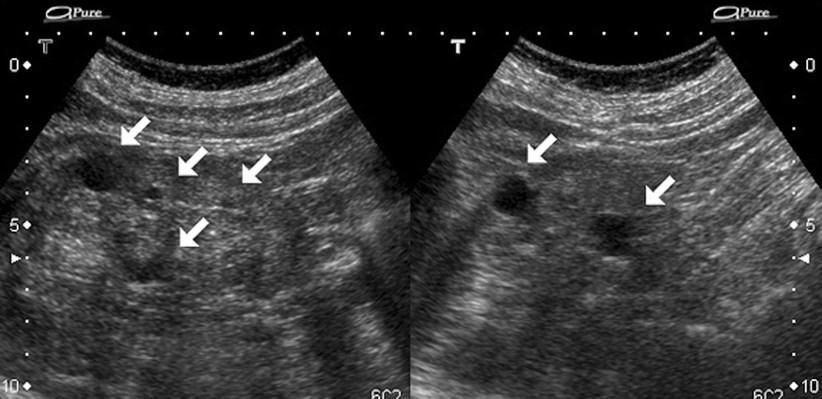
Fig. 3.3
Simple cyst. A 76-year-old woman on long-standing dialysis. Sonography shows bilateral, multiple small simple cysts (arrows), and highly echogenic renal parenchyma. These cysts are secondary to acquired cystic renal disease
Computed Tomography (CT)
Well-defined, homogeneous hypodense lesion showing sharp interface with adjacent renal parenchyma and water content (density measurement <20 HU). No wall thickening, calcification or enhancement accompanies.
Magnetic Resonance Imaging
Round or oval lesions with signal intensity of simple fluid on all sequences resulting in low signal on T1 weighted and high signal on T2-weighted images.
No internal structures, wall thickening, or septa should be appreciable.
After gadolinium administration, there is no enhancement.
Pathology
Gross: Simple cysts are most commonly found in the cortex and are filled with clear fluid (Fig. 3.4).
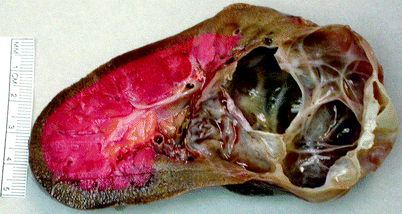
Fig. 3.4
Simple cortical cyst. Simple cortical cyst may be single, multiple, widespread, or localized. It is usually located in the cortex and is filled with clear serous fluid. The lesion shown was a conglomerate of closely situated simple cysts (Image courtesy of Richard Naturale, M.D.)
Microscopy: The cyst lining is translucent and glistening and is composed of cuboidal or flattened epithelium (Fig. 3.5). The cyst wall may calcify.
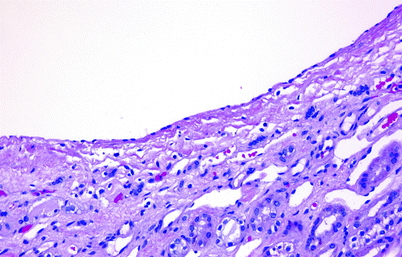
Fig. 3.5
Simple cortical cyst. The cyst is typically lined by flattened epithelium. Sometimes no lining epithelium is identified. The wall is often thick and fibrotic and is occasionally calcified
Differential Diagnosis
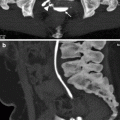
The obstructed upper moiety calices of a duplicated collecting system can mimic an upper pole cyst in a child (Fig. 3.6).
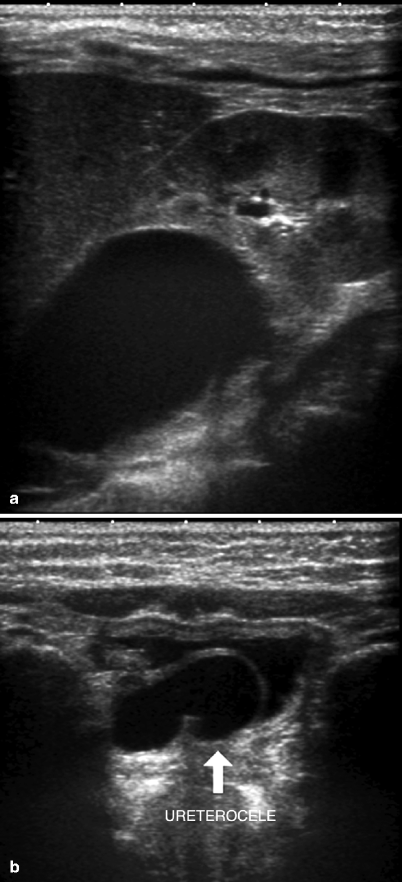
Fig. 3.6
Duplicated renal collecting system with upper pole obstruction. A 2-year-old girl with anechoic right renal upper pole cystic structure of 3 cm that mimics a simple cyst (a). The ureter is dilated, and a ureterocele (arrow) is seen on the right side of the bladder (b)
Calyceal diverticulum. Usually, chronic obstruction of a calyx causes a diverticulum that is indistinguishable from a cyst, but delayed nephrographic phase will fill the diverticulum with contrast (Figs. 3.7 and 3.8).
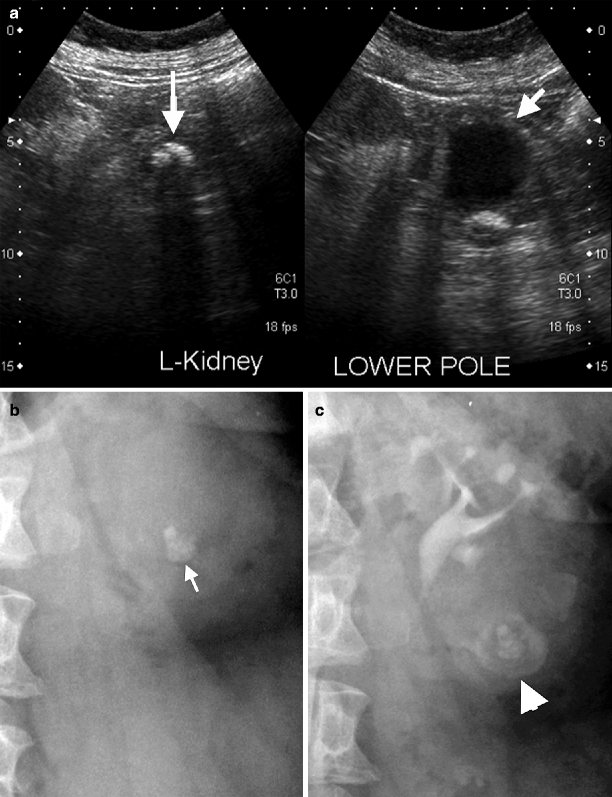
Fig. 3.7
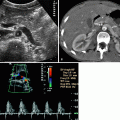
Calyceal diverticulum. (a) Sonography shows a small lower pole calyceal cyst that mimics a cyst (short arrow) adjacent to a big stone (long arrow). (b) Plain film shows a big lower pole stone (arrow). (c) Contrast pooling in the lower pole of left kidney adjacent to the renal stone confirms the diagnosis of calyceal diverticulum (arrowhead)
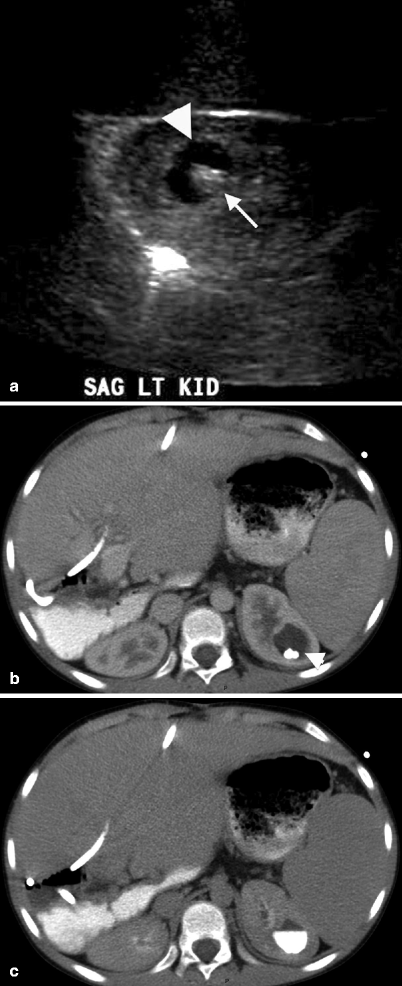
Fig. 3.8
A cystic lesion (arrowhead) is seen on the superior pole of the left kidney with anechoic appearance and a hyperechogenic focus (arrowhead) which was assumed to be wall calcification or stone (a). CT image of same patient at nephrogram phase reveals hypodense cystic lesion (arrowhead) which contains stones (b). Delayed nephrographic phase CT depicts the filling of the presumed cyst with contrast material confirming the diagnosis of a calyceal diverticulum (c)
Vascular abnormalities such as aneurysms and pseudoaneurysms may also mimic simple cysts with anechoic appearance on US. Doppler US evaluation may diagnose these lesions (see Fig. 4.21).
Pearls and Pitfalls
Simple cysts can be detected on plain films if they are big enough to create a separate density.
The best imaging method to confirm a renal simple cyst is ultrasound.
Calyceal diverticulum can mimic a simple renal cyst.
A peripelvic cyst can also mimic a simple renal cyst.
Parapelvic Cysts
General Information
Their origin is likely lymphatic or develop from embryologic rests.
Most of these cysts are asymptomatic although they may cause hematuria, hypertension, and hydronephrosis.
Imaging
Intravenous Pyelography
Parapelvic cysts may cause elongation and displacement in renal calices and stretching infundibuli.
IVP may be useful to differentiate multiple parapelvic cysts from hydronephrosis by demonstrating absence of caliceal dilatation.
Ultrasonography
Well-defined anechoic masses in renal sinus that do not communicate with the renal collecting system.
Typically ellipsoid in shape and may mimic hydronephrosis since they are located more centrally within the renal sinus (Fig. 3.9).
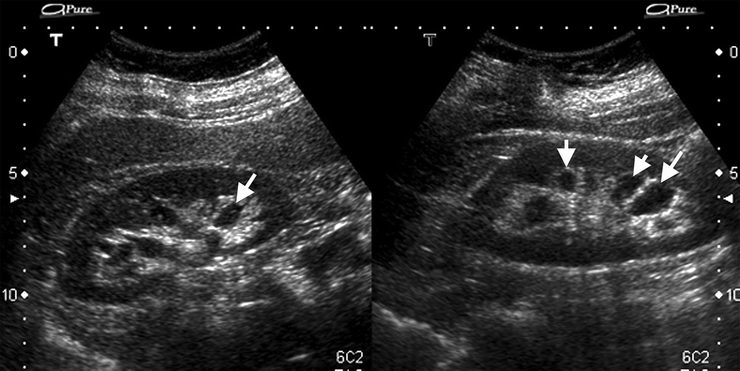
Fig. 3.9
Parapelvic cysts. Sonography of a 59-year-old woman with bilateral parapelvic small cysts (arrows)
Computed Tomography
Parapelvic cysts show similar CT findings as cortical cysts; however, they are located in renal sinus and not the renal cortex.
Parapelvic cysts do not show contrast enhancement.
Differential Diagnosis
Multiple parapelvic cysts usually mimic hydronephrosis and vesicoureteric reflux. On US, CT, and MRI dilated calices may be shown to communicate with renal pelvis which is diagnostic of hydronephrosis. However, parapelvic cysts do not communicate with renal pelvis.
Postvoid imaging is important to differentiate parapelvic cysts from vesico-ureteric reflux. Parapelvic cysts will not disappear on postvoid images.
Pearls and Pitfalls
Parapelvic cysts do not communicate with the renal collecting system.
These cysts may result in hematuria, hypertension, and hydronephrosis.
Complex Cyst
General Information
A simple cyst with hemorrhage, infection, or debris is called a complex cyst; however, a cystic RCC may present as complex cystic mass.
Bosniak classification is widely accepted as a useful guide in the management of complex renal cysts. An exception to the rule is a hydatid renal cyst in which nonspecific (wall thickening or calcification, internal echoes, and avascular solid components) or specific findings (membrane detachment and daughter cysts) can be detected.
Imaging
Ultrasonography
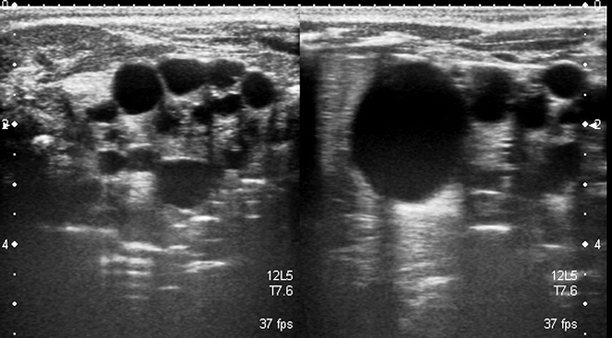
A cyst with a thickened or calcified wall with septae, internal echoes, or mural nodules is described as a complex cyst (Fig. 3.10).
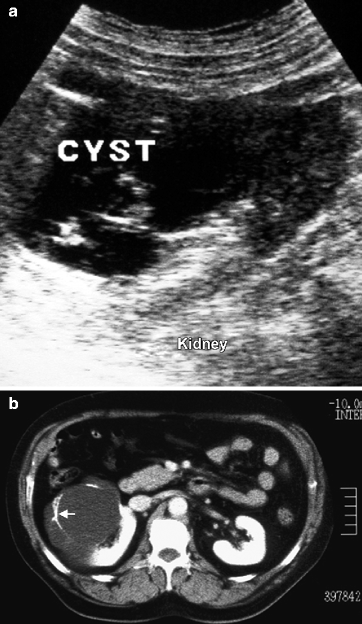
Fig. 3.10
Complex cyst. (a) Sonography shows a cyst with thick, irregular, and calcified multiple septae. (b) The extent of calcification in septae (arrow) is better shown by CT
Tissue harmonic imaging is helpful to eliminate “pseudo” or “dirty” echoes and confirm complex cystic lesion.
Complex cysts should always be evaluated by combined US, color Doppler US, and CT or MRI to detect any vascularity of their solid contents, the extent of calcification, and the density of the cystic components (Fig. 3.11).
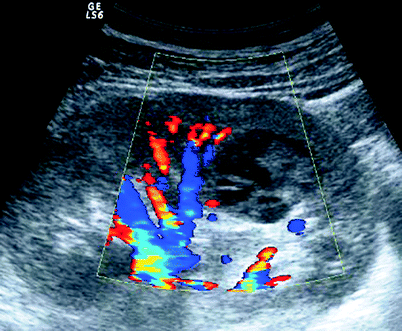
Fig. 3.11
Complex cyst. Color Doppler US demonstrates a complex cyst with no vascularity
CT-based Bosniak classification may not be strictly adapted on US.
Thickened wall, mural nodularity, and thickened (>2 mm) and vascularized septa are sonographic malignancy indicators on US.
Aspiration cytology for complex cysts is not regarded to be reliable since it may have a high false negativity.
Computed Tomography
Density of fluid content may be >20 HU on noncontrast CT.
Complex cyst may demonstrate wall thickening, calcification, and presence of septa.
Infected cysts may contain air density.
Benign complex cysts have septal thickening of <2 mm and absence of contrast enhancement of septae.
Bosniak classification was firstly used with CT imaging features of cysts, but then, it was adapted in US and MRI.
Bosniak Cassification on CT is summarized as follows:
Category I: Thin wall; no septa, calcification, solid component, or enhancement; water attenuation (Fig. 3.12)
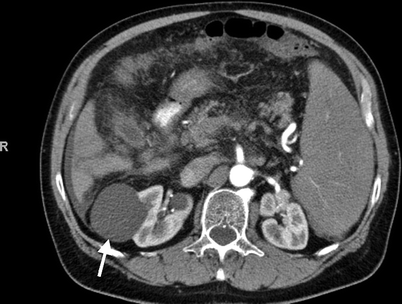
Fig. 3.12
Bosniak category I. Axial contrast-enhanced CT demonstrates a low density simple cyst (arrow) without contrast enhancement (From Strang JG, Dogra V. Body CT secrets. Philadelphia: Mosby/Elsevier; 2007. Reprinted with permission)
Category II: Few hairline-thin septa with or without enhancement; fine calcification in the wall or septa; ≤3 cm homogeneously high attenuating masses that are sharply marginated and do not enhance (Figs. 3.13 and 3.14)
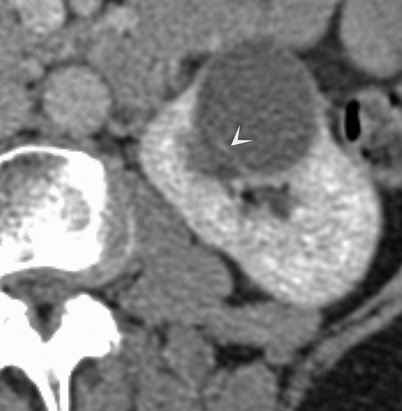
Fig. 3.13
Bosniak II cyst with a thin (<1 mm) septa (arrowhead) (From Strang JG, Dogra V. Body CT secrets. Philadelphia: Mosby/Elsevier; 2007. Reprinted with permission)
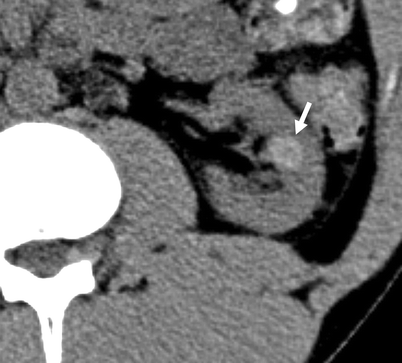
Fig. 3.14
Bosniak II cyst. A hyperdense (hemorrhagic) cyst (arrow) in the left kidney on a non-enhanced CT (NECT) scan (From Strang JG, Dogra V. Body CT secrets. Philadelphia: Mosby/Elsevier; 2007. Reprinted with permission)
Category II F: Multiple hairline-thin septa with or without enhancement; minimal smooth thickening of wall or septa that may show no perceived enhancement; no enhancing soft tissue components; intrarenal nonenhancing high-attenuation renal masses (>3 cm) (Fig. 3.15)
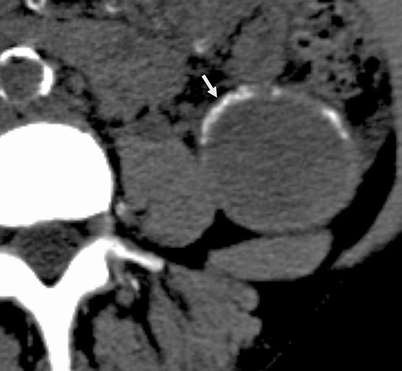
Fig. 3.15
Bosniak II F cyst. NECT image of the left kidney reveals a 4.8 cm cystic lesion with thick, peripheral calcification. Size >3 cm and presence of thick peripheral calcification rules out a Bosniak II category, and is called a Bosniak IIF cyst (From Strang JG, Dogra V. Body CT secrets. Philadelphia: Mosby/Elsevier; 2007. Reprinted with permission)
Category III: Thickened irregular or smooth walls or septa, with measurable enhancement (Fig. 3.16)
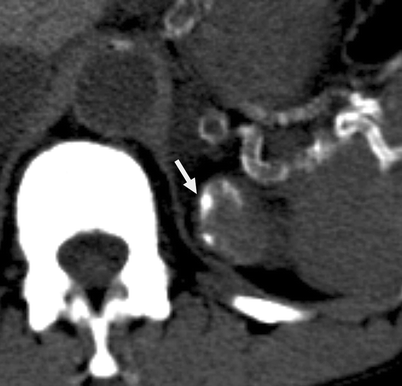
Fig. 3.16
Bosniak III cyst. Cystic lesion arising from the superior pole of the left kidney with thicker and nodular calcification (arrow) (From Strang JG, Dogra V. Body CT secrets. Philadelphia: Mosby/Elsevier; 2007. Reprinted with permission)
Category IV: In addition to criteria of category III, enhancing soft tissue components adjacent to, or separate from the wall or septa (Fig. 3.17)
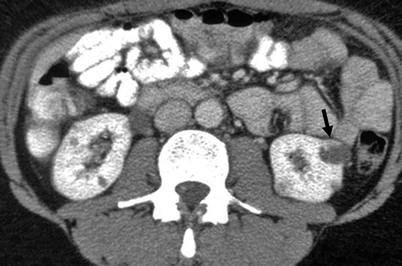
Fig. 3.17
Bosniak IV cyst. A patient with known history of von Hippel-Lindau disease with multiple bilateral renal lesions. A cystic lesion in the left renal midpolar region with an enhancing mural nodule (arrow) is suggestive of renal cell carcinoma (From Strang JG, Dogra V. Body CT secrets. Philadelphia: Mosby/Elsevier; 2007. Reprinted with permission)
Magnetic Resonance Imaging
Complicated cysts are hyperintense on T1 and T2 weighted images due to hemorrhagic or proteinaceus content.
Hemorrhagic cysts do not show contrast enhancement, whereas infected cysts may show enhancement.
MRI is insensitive in detection of calcification.
Differential Diagnosis
Aspiration cytology for complex cysts is not regarded to be reliable since it may have a high false-negative results.
Cystic RCCs differ from complicated cysts by showing poor defined walls and intense enhancement after contrast administration on MRI.
Pearls and Pitfalls
Benign criteria for complicated cysts are septa thickening <2 mm and absence of contrast enhancement of septa.
MRI has increased ability to detect cyst septa in comparison with CT.
Multicystic Dysplastic Kidney
General Information
It is a developmental entity characterized by nonfunctioning kidney with multiple cysts of varying size is called a multicystic dysplastic kidney (Fig. 3.18).