(1)
Princess Elizabeth Hospital Le Vauquiedor St. Martin’s Guernsey, Channel Islands, UK
(2)
Brighton and Sussex Medical School, Brighton, England UK
Abstract
Several terms have been applied to the condition of fibrocystic change, including mammary dysplasia, fibrocystic disease, cystic mastopathy and cystic hyperplasia.
Learning Points
Fibrocystic change replaced the term fibrocystic disease.
Fibrocystic change consists of cysts lined by attenuated cells or apocrine epithelium.
Fibrocystic change with apocrine epithelium is a common abnormality present in benign and cancerous breast.
The cause of fibrocystic change is unknown but may be related to hormonal abnormality or dietary factors.
Simple cysts are adequately managed by aspiration under ultrasound guidance.
Cytological assessment of cyst contents is only necessary if the contents are bloodstained.
Complex cysts are thick walled and have thick septae, intracystic masses or other solid component.
Recurrent cysts can be managed by aspiration but will require needle core biopsy or excision if bloodstained or if there is a persistent mass.
There is no consensus regarding the risk of breast cancer in patients with fibrocystic change but generally considered to be very low.
Chromosomal abnormalities in apocrine epithelium suggest possible precursor of apocrine carcinoma.
7.1 Fibrocystic Change
7.1.1 Background and Clinical Features of Fibrocystic Change
Several terms have been applied to the condition of fibrocystic change, including mammary dysplasia, fibrocystic disease, cystic mastopathy and cystic hyperplasia.
The term fibrocystic disease was laid to rest in 1985 at a consensus meeting held by the Cancer Committee of the College of American Pathologists in favour of fibrocystic change (Hutter et al. 1986). This meeting was prompted by complaints from women who were paying high insurance premiums following the diagnosis of fibrocystic disease. The participants at the meeting agreed that if the term fibrocystic change was used, the associated benign epithelial proliferations should be stated to assist in assessing individual risk. In 1998, Fitzgibbons et al. published the updated version of the consensus definitions which included categories of risk based on pathologic diagnosis (Fitzgibbons et al. 1998). In this book, the term fibrocystic change is applied to lesions consisting of cysts, some of which are lined apocrine epithelium, with or without associated fibrosis. Although fibrocystic change can be present as the dominant disease process, in the majority of cases the proliferation is identified in breast tissue excised for other benign or malignant disease (Foote and Stewart 1945; Wellings and Alpers 1987). Benign epithelial proliferations associated with fibrocystic change include intraductal papillomas, fibroadenoma, duct ectasia, radial scar, sclerosing adenosis (apocrine adenosis) epithelial hyperplasia of usual type and many more. Fibrocystic change is also present in ‘normal’ breast tissue (Frantz et al. 1951), is rare before the age of 20, becomes more prevalent in perimenopausal women and the microscopic lesion persists in postmenopausal women (Wellings and Alpers 1987). Fibrocystic change can present symptomatically with palpable mass or detected during mammographic screening.
7.1.2 Possible Aetiological Factors and Pathogenesis of Fibrocystic Change
Because of the distinct appearance of the apocrine epithelium which makes it prominent among other proliferations, theories abound as to the possible aetiological factors of fibrocystic change. There is no consensus as to whether the characteristic ‘pink epithelium’ of Lendrum (1945), which microscopically and ultrastructurally resembles the apocrine sweat glands (Ozzello 1971), originates from the sweat gland (Haagensen 1986), is native to the breast and metaplastic (Ahmed 1975) or represents epithelial degeneration (Dawson 1932). The eosinophilic appearance of apocrine epithelium is due to the presence of excess mitochondria (Ahmed 1975). Although the metaplastic theory is generally accepted, this has been challenged by the detection of cells that are positive for gross cystic disease fluid protein-15 (GCDFP-15), a marker of apocrine cells, in developing fetal breast tissue (Viacava et al. 1997).
In addition to GCDFP-15, GCDFP-24 has been identified in fibrocystic change. These proteins are significant constituents of the proteins present in the fluid aspirated from the cysts in fibrocystic change (Haagensen et al. 1979). GCDFPs can be detected by immunohistochemistry in apocrine epithelium but not in normal breast tissue. GCDFP-15 has the highest concentration in the cystic fluid and this protein has been extensively investigated in several tumours to assess its specificity for apocrine epithelium. The initial study by Mazoujian et al. (1983), which was later followed by a publication from Wick and colleagues (1989), confirmed GCDFP-15 as a specific marker for apocrine differentiation.
Simard and colleagues (1989, 1990) demonstrated that androgens stimulate GCDFP-15 and GCDFP-24 production in breast cancer cell lines, whereas oestrogens inhibit the production of these proteins. Based on these observations, Haagensen (1991) postulated that the selector genes that control apocrine metaplasia may, as part of this process, also govern co-ordinated expression of GCDFPs, and the hormonal modulation of these proteins appears to be under similar mechanisms, with androgens enhancing synthesis and oestrogens having an inhibitory effect. The results of these hormonal effects can be illustrated by immunohistochemical staining. Apocrine epithelium expresses androgen receptors, but lacks oestrogen and progesterone receptors (Gatalica 1997; Selim and Wells 1999). Although apocrine epithelium lacks oestrogen-α receptor, Shaaban et al. (2003) demonstrated expression of oestrogen-β receptor in apocrine metaplasia and other benign breast epithelium, which may have a bearing on carcinogenesis. Apocrine carcinomas are oestrogen and progesterone receptors negative.
Because the condition peaks during the perimenopausal period, several hormonal abnormalities have been implicated in the pathogenesis of fibrocystic change, including hyperprolactinaemia, increased oestrogen levels, reduced progesterone levels and excess thyroid hormone activity (Drukker and deMendonca 1987). However, the changes in the hormonal levels have not been constant in all patients to make this a definite aetiological factor.
Ingestion of excess tea, coffee, chocolates and certain cola drinks, which produce methylxanthines, has been implicated in the development or exacerbation of fibrocystic change. Minton et al. (1979a) proposed that methylxanthines inhibit cyclic adenosine monophosphate phosphodiesterase and cyclic guanosine monophosphate phosphodiesterase action, thereby increasing tissue levels of adenosine monophosphate and guanosine monophosphate compounds, which were reported to be present in women with fibrocystic change. As a follow-on study, Minton and colleagues (1979b) reported disappearance of symptoms in 65 % of 20 women who abstained from taking methylxanthines for 1 to 6 months. A separate case-controlled study, which included 634 women with fibrocystic change and 1,066 controls, also reported a positive association of fibrocystic change with caffeine consumption (Boyle et al. 1984). Women who consumed 31–250 mg of caffeine a day had a 1.5-fold increased risk of developing fibrocystic change and those who drank more than 500 mg a day had a 2.3-fold increased risk. This study also reports high caffeine consumption in women with atypical lobular hyperplasia, sclerosing adenosis and ‘papillomatosis’, lesions that were part and parcel of ‘fibrocystic disease’ prior to the 1985 consensus meeting. Inclusion of these confounding factors does not give a true effect of caffeine, if any, on the pathogenesis of fibrocystic change.
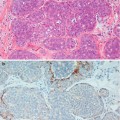
Haagensen (1991) postulated that the microscopic lesion that is the progenitor of gross cystic disease of the breast is apocrine metaplastic change of the epithelium in the terminal duct–lobular units. Secretions from the apocrine cells expand the lobules and this progresses into apocrine-lined microcysts, which further expand into grossly apparent cysts. This process was succinctly illustrated by Warner and colleagues (Fig. 7.1). Warner et al. (1998) further expanded this theory and indicated that the microcysts were caused by unfolding of lobules, based on the previous work by Wellings and Alpers (1987). Wellings and Alpers performed subgross analysis of fibrocystic change on 186 post-mortem breasts and 107 breasts with cancer. The investigators observed that fibrocystic change with apocrine metaplasia arose in terminal duct–lobular units and hypothesised that the cysts are created by ‘unfolding of the lobules’. As time progresses, the small cysts coalesce to create larger cysts. In the older women the cysts were larger and lined by flat epithelium, and the luminal contents were more voluminous, indicating an aging process.
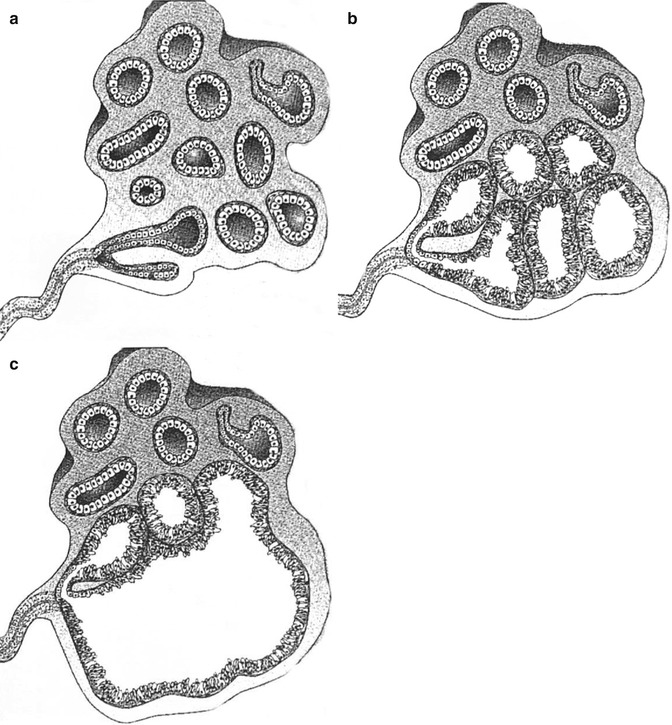

Fig. 7.1
Schematic drawings of apocrine metaplastic change in terminal duct–lobular unit. (a) Normal terminal duct–lobular unit shows acini lined by cuboidal epithelium. (b) Many acini within the terminal duct–lobular unit are now lined by columnar epithelium and are dilating, presumably because of fluid production by this apocrine metaplastic epithelium. (c) Several adjacent acini have fused to form a larger cystic space (From Warner JK, Kumar D, Berg WA (1998) Apocrine metaplasia: mammographic and sonographic appearances. Am J Roentgenol 170: 1375–1379, Fig. 4A, B, C. Reprinted with permission from the American Journal of Roentgenology)
7.2 What Is the Stimulus that Evokes the Metaplastic Process?
Slack (1986) believed that epithelial metaplasia is a single-step process without an intermediate stage. He hypothesised that metaplasia is caused by a master selector gene that distinguishes between various tissues and co-ordinates changes in cell kinetics, responses to growth factors and hormones. Haagensen (1991) expanded this theory further and formulated a model of the possible interacting processes, which eventually lead to breast cancer with apocrine features as illustrated in Fig. 7.2.
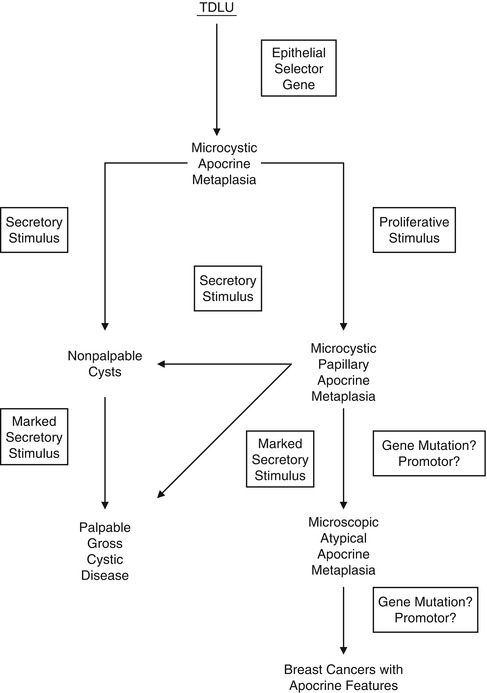

Fig. 7.2
Diagram of possible pathways for breast epithelial changes that result in breast gross cystic disease or in breast carcinoma with apocrine features. TDLU terminal duct–lobular unit (Reproduced with permission from Lippincott Williams & Wilkins)
7.3 Molecular Pathology of Fibrocystic Change
Apocrine epithelium is an inherent feature of fibrocystic change and the presence of apocrine cells in a breast biopsy is generally considered by pathologists to be a reassuring feature of benignity. However, this view is changing with time due to detailed study of this lesion and application of molecular markers. There is molecular evidence that some benign forms of apocrine epithelium may be potentially neoplastic.
Expression of C-myc and ras proteins has been reported with a high prevalence in apocrine epithelium with papillary proliferation (Agnantis et al. 1992). As both C-myc and ras oncoproteins are expressed in several cancers, their presence in papillary apocrine epithelium suggests possible indicators of malignant change.
One of the major changes that occur in carcinogenesis is loss of heterozygosity (LOH), and this was assessed by Washington et al. (2000) on 32 breast specimens with fibrocystic change. Twenty of the patients were postmenopausal. Fourteen lesions of apocrine metaplasia adjacent to cancer were also assessed for LOH. LOH was assessed on 9p, 11p, 13q, 16q, 17p and 17q because these chromosomal loci show a high frequency of LOH in breast tumours. LOH was detected in 6/27 normal terminal duct–lobular units, 4/23 adenosis, 4/21 ductal hyperplasia and 10/19 apocrine metaplasia. Seven out of 14 specimens of apocrine metaplasia adjacent to carcinoma also showed LOH. In all seven cases the carcinoma and apocrine epithelium shared LOH at one or more loci. Because of the high frequency of LOH in apocrine metaplasia in non-neoplastic cases, the authors hypothesised that apocrine epithelium could be derived from genetically altered cells, which also give rise to an adjacent carcinoma. LOH in some apocrine metaplasia and adjacent carcinoma also suggested a common clonal precursor.
In a separate study, comparative genomic hybridisation (CGH) was applied to benign apocrine hyperplasia, apocrine ductal carcinoma in situ (DCIS) and apocrine invasive carcinoma to assess the presence of genetic alterations in these lesions (Jones et al. 2001). The mean number of genetic alterations (chromosomal gains and losses) in apocrine hyperplasia was 4.1 (n = 10), apocrine DCIS, 10.2 (n = 10) and invasive apocrine carcinoma, 14.8 (n = 4). There was overlap in the genetic alterations in apocrine papillary hyperplasia, DCIS and invasive carcinoma. Losses at 1p, 16q and 17q and gains at 2p and 13q were detected in multiple cases of all three types of proliferations (hyperplasia, in situ and invasive carcinoma). Based on the widely held view that there is progression from hyperplasia through in situ change to invasive carcinoma, the authors suggested that the chromosomal alterations might be early changes in the carcinogenesis of apocrine breast cancer. The detection of genetic alterations in papillary apocrine proliferation also implied that a proportion of these lesions might be clonal neoplasms. The overlap of some chromosomal abnormalities with apocrine DCIS and invasive carcinoma proposed apocrine papillary hyperplasia as a putative non-obligate precursor of apocrine carcinoma.
7.4 Radiological Features of Fibrocystic Change
7.4.1 Mammography
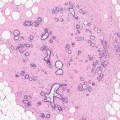
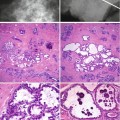
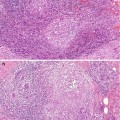
Although cysts are usually present in association with other symptomatic or screen-detected lesions, they can be the main pathological abnormality at symptomatic presentation or as a screen-detected lesion. Cysts can appear as round, oval or well-circumscribed masses (Fig. 7.3). If present in dense breasts, cysts may be partially obscured or mammographically invisible (Heywang-Köbrunner et al. 2001), and in fatty breasts, the cysts can exhibit the halo sign (Tabár and Dean 1985). Warner and colleagues (1998) carried out a retrospective study on mammographic and sonographic features of 17 lesions in which apocrine metaplasia constituted 50 % of the lesions. The diagnosis was made on needle core biopsies in 13 patients and on fine needle aspiration cytology in the remaining four. The mean age of the patients was 57 years (range 37–95 years). Nine of the lesions were screen-detected. The average size of the lesions was 12.8 mm (range 6–24 mm). Fifteen lesions were mammographically of equal density to the surrounding breast tissue. Two lesions had lower density than the surrounding parenchyma and none of the lesions was of higher density than the adjacent breast tissue. Calcification was not a common feature and this was only present in a single lesion. Ten lesions exhibited microlobulated borders, five had macrolobulated borders and two had circumscribed oval borders. Eleven of the 17 lesions decreased in size at the time of needle core or fine needle aspiration, confirming their cystic nature. Most of the lesions showed variable resolution or decrease in size on follow-up mammography. On histological examination, 9/17 lesions had isolated focal apocrine metaplasia and predominantly apocrine metaplasia in 8/17 cases. The latter cases had associated fibrosis (four cases), sclerosing adenosis (three cases) and intraductal epithelial hyperplasia (single case).
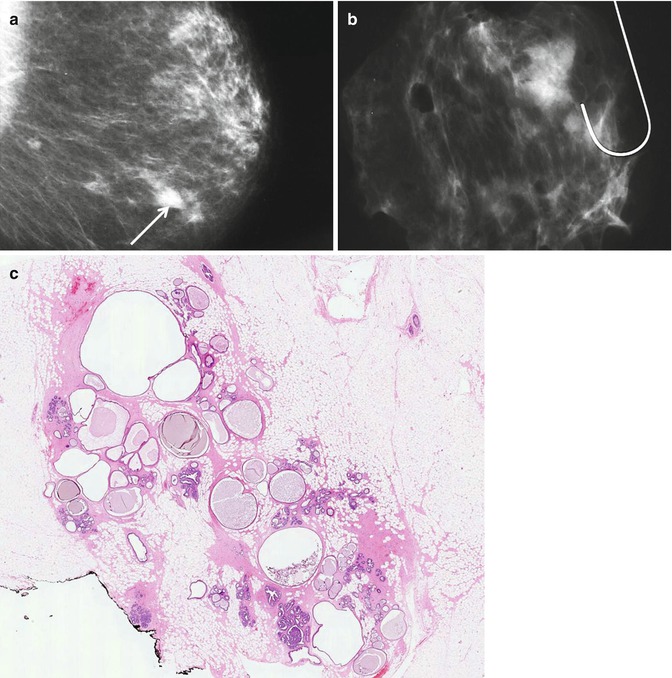

Fig. 7.3
(a) The mammogram shows a new, irregular, soft tissue density in a 57-year-old woman graded as R3 (arrow). Ultrasonography showed multiple cystic spaces with associated fibrous tethering. The needle core biopsy was not representative and the lesion was excised. (b) The specimen X-ray shows an ill-defined soft tissue density with no calcification. (c) Whole mount section of the excised lesion shows fibrocystic change consisting of multiple cystically dilated duct–lobular units, some containing featureless eosinophilic secretions
When Warner et al. (1998) compared the radiological and histological features, they noted that the mammographic microlobulated and macrolobulated pattern corresponded with lobulated margins of a dilated terminal duct–lobular unit on histology. Despite the correlation of mammographic and sonographic features with the histological appearances, the authors were reluctant to recommend the diagnosis of apocrine metaplasia on radiological appearances alone, due to the small size of the sample. Moreover fibrocystic change is invariably associated with other disease processes and a radiological diagnosis without a biopsy may not be safe. Kushwaha et al. (2003) did not find the mammographic features of fibrocystic change to be diagnostic when they performed a mammographic–pathologic correlation of apocrine metaplasia diagnosed using vacuum-assisted stereotactic needle core biopsy. The pattern of calcification was heterogeneous, as were the soft tissue abnormalities. However, Lanyi (2003) describes a ‘tea cup phenomenon’ as pattern of calcification unique to cystic lesions. This mammographic abnormality occurs in 90° lateral views due to sedimentation of the calcification which is heavier than the fluid in the cyst.