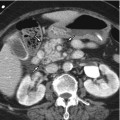
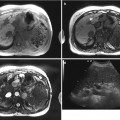
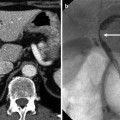
Epithelial
Non-epithelial
Neoplastic
Benign
Serous cystadenoma
Lymphangioma
Mucinous cystadenoma
Borderline
IPMN with low- or intermediate-grade dysplasia
Sarcoma, cystic
IPMN with high-grade dysplasia
MCN with low- or intermediate-grade dysplasia
MCN with high-grade dysplasia
Neuroendocrine tumor grade 1 or 2, cystic
Malignant
IPMC with an associated invasive carcinoma
MCN with an associated invasive carcinoma
Serous cystadenocarcinoma
Solid pseudopapillary neoplasm
Ductal adenocarcinoma, cystic
Neuroendocrine carcinoma, cystic
Pancreatoblastoma, cystic
Cystic metastatic epithelial neoplasm
Nonneoplastic
Lymphoepithelial cyst
Pancreatitis-related pseudocyst
Retention cyst
Parasitic cyst
Enterogenous cyst
Mucinous nonneoplastic cyst
Paraampullary duodenal wall cyst
Endometrial cyst
19.1 Terminology
Because the morphology of the cysts is important to differentiate among cystic pancreatic tumors, radiologists should be familiar with the terminology which is commonly used for the description of cyst morphology. First, cystic pancreatic lesions are classified according to the number of compartments divided by the internal septum. By definition, when there is one cyst, it is defined as unilocular; when there are two to six cysts, oligolocular; and when there are seven or more, multilocular. Classification of cyst size is also important. If all cysts are less than 2 cm in size, they are called microcystic lesions, while if there is at least one cyst greater than or equal to 2 cm, it is referred to as a macrocystic lesion. In addition to the number and size of the cysts, other morphologic features such as outer contour (smooth vs. lobulated), the presence of communication between the cyst and the pancreatic duct, mural nodule, calcification, and central scar should also be considered. Cystic pancreatic lesions can be associated with different patterns of pancreatic ductal dilatation: diffuse, upstream, and downstream dilatation. Finally, characteristic shapes of the cystic lesions such as honeycomb, pleomorphic, and clubbed fingerlike appearance are sometimes an important clue for the differentiation.
19.2 Intraductal Papillary Mucinous Neoplasm (IPMN)
IPMN is pathologically characterized by papillary proliferation of ductal epithelium in the main or branch duct, association with ductal dilatation, and various mucin productions. Based on the papillary components, IPMN can be subdivided into gastric-foveolar, villous-intestinal, pancreatobiliary, and oncocytic subtypes. These subtypes explain the variable malignant behavior of the IPMN as well as the association identified between IPMN and synchronous or metachronous extrapancreatic malignant neoplasms (the stomach, colon, liver, etc.) in up to 29 % of cases. Since growths affecting the pancreatic main duct are associated with a higher malignant potential, IPMNs are subcategorized clinically into main duct type, branch duct type, and combined type. The 2010 WHO classification subcategorizes the IPMN according to their malignant transformation into IPMN with low or intermediate dysplasia, IPMN with high-grade dysplasia, and IPMN with an associated invasive carcinoma. In addition to pancreatic intraepithelial neoplasia (PanIN), IPMN is considered to be the most important precursor lesion of ductal adenocarcinoma in the pancreas. The risk of malignancy being present at the time of diagnosis increases with older age, presence of symptoms, involvement of the main pancreatic duct, dilation of the main pancreatic duct over 10 mm, the presence of mural nodules, and size over 3 cm for branch duct type IPMN. Imaging findings of IPMNs depend on their morphologic subtypes. Main duct type IPMN reveals diffuse or focal dilatation of the main pancreatic duct (≥1 cm). Branch duct type IPMN typically exhibits focal, multicystic dilatation of one or more branch duct with normal or downstream main ductal dilatation. On cross-sectional image, one dilated branch duct appears as a fingerlike cystic lesion connecting to the main duct. Multiple dilated branch ducts show pleomorphic appearance with different (round, oval, and tubular) shapes of cystic lesions. To distinguish from other cystic tumors, it is essential to identify a ductal connection between the lesion and pancreatic ductal system. In addition to the two IPMN types described above, there is also a mixed type, which spreads into the main duct from the branch ducts and vice versa. Differentiation among the subtypes of IPMN is of pivotal importance because the malignant potential of main duct type IPMN is relatively high at 57–92 %, whereas the probability of branch duct type IPMN is much lower.
19.3 Serous Cystic Neoplasm (SCN)
SCNs are microscopically characterized by cysts lined by glycogen-rich, cuboidal cells, filled with clear and thin serous fluid with richly vascular collagenous or hyalinized stroma. SCNs previously referred to “microcystic adenomas” while mucinous cystadenomas to “macrocystic adenomas.” Therefore, the characteristic imaging feature of SCNs is a honeycomb pattern with central scar or central calcification, the typical imaging findings of serous microcystic adenomas. However, with rapid advancements and increased use in imaging techniques as well as with recent increased knowledge of pancreatic SCNs, SCNs are further categorized into five different subtypes according to their macroscopic appearances: serous microcystic adenomas, the typical and traditional SCN; serous oligocystic ill-defined adenoma (SOIA); von Hippel-Lindau (VHL)-associated cystic neoplasm; solid serous adenoma; and serous cystadenocarcinoma. Imaging findings of SCNs reflect a variety of macro- and microscopic findings and can have variable appearances from compactly solid hypervascular to clearly unilocular cystic pattern with some mixture both radiologically and pathologically. Serous microcystic adenomas can be misdiagnosed as a solid enhancing tumor if the fibrous septa predominate. T2-weighted MRI or endoscopic US can be usually helpful to confirm the microcystic nature of the lesion. A spongelike pattern in serous microcystic adenoma is found if the cysts increase in size peripherally. The oligocystic variant, known as SOIA, comprises just a few larger (>2 cm) cysts without central scarring or calcification, therefore making morphologic differentiation from mucinous cystadenoma difficult. Differential points of SOIA from MCN include lobulating outer margin. The VHL-associated serous adenoma appears as multiple serous cystic lesions distributed throughout the entire pancreas. Other organ involvement such as brain, retinal, and spinal cord hemangioblastomas; renal cysts and renal cell carcinoma; pheochromocytomas can be combined. Solid serous adenoma manifesting itself as a hypervascular solid lesion without cystic portion is radiologically confused with true solid tumors such as neuroendocrine tumors or solid pseudopapillary tumors. Serous cystadenocarcinoma is very rare and only less than 30 cases have been reported. Imaging findings of serous cystadenocarcinoma are similar to those of serous microcystic adenoma except gross evidence of invasiveness.
19.4 Mucinous Cystic Neoplasm (MCN)
Mucinous cystic neoplasm (MCN) occurs almost exclusively in women, showing no communication with the pancreatic ductal system and composed of columnar, mucin-producing epithelium, supported by ovarian-type stroma. According to the grade of intraepithelial dysplasia, tumors may be classified as MCN with low- or intermediate-grade dysplasia, MCN with high-grade dysplasia, and MCN with an associated invasive carcinoma. Radiologically, MCN appears as a single lesion with unilocular or multilocular cysts as well as internal septa. The thickness of the cyst wall and fibrous septa is almost uniform and the outer contour is smoother than in SCN. In contrast to SCN, incidental calcifications appear rather peripherally in a linear, eggshell pattern. Hemorrhage within the cyst is indicative of this tumor. MCN generally exhibits no connection to the pancreatic duct and rather displaces the duct. Solid mural nodules, cyst dimensions of >6 cm, and peripheral calcification are well-accepted criteria for malignancy.
19.5 Cystic Degeneration of Solid Pancreatic Tumor
Solid pancreatic tumors such as solid pseudopapillary neoplasm (SPN) and pancreatic ductal adenocarcinoma may undergo cystic degeneration, masking its originality as a solid tumor. SPT is found almost in young women with a mean age of 30 years. SPT consists largely of solid components alternating with pseudopapillary formations of tissue, supported by strands of vascular, hyaline tissue. Radiologically, it appears as a partial cystic mass surrounded by a thick, irregular, enhancing capsule. A 2–10 % of pancreatic NET may also show cystic change. A cystic form of NET tends to have favorable prognosis. Cystic feature of solid pancreatic tumors may result due to necrosis, hemorrhage, and degeneration of tumor cells.
19.6 Other Cystic Lesions
Non-epithelial cystic tumors such as lymphangioma can be rarely developed in the pancreas. In addition, nonneoplastic cystic lesions such as lymphoepithelial cyst and retention cyst may occur in the pancreas. Lymphoepithelial cyst is lined by mature squamous epithelium and surrounded by lymphoid tissue and is usually filled with keratinized material. Although lymphoepithelial cyst is categorized as a pancreatic cystic lesion, it is usually located at peripancreatic or extrapancreatic area because it is known to be developed from epithelial remnants in the lymph node outside of the pancreas. Radiologically, it shows a lobulated margin and has slightly hyperdense fluid due to keratinized material. A pancreatic retention cyst is defined as a segment of a pancreatic duct that is cystically dilated as a consequence of duct obstruction. Duct obstruction is caused by fibrous strictures, mucin plugs, calculi, tumors, or neoplastic epithelial proliferations such as PanIN. A retention cyst appears as a small cystic lesion communicating with the pancreatic duct as a consequence of duct obstruction. However, it cannot be confidently diagnosed preoperatively. Epidermoid cyst in intrapancreatic accessory spleen may also mimic cystic pancreatic tumors. However, typical location (pancreatic tail) and enhancement pattern of remnant solid component which is the same or similar to that of the main spleen on CT or MRI can be important clues for differentiating from other cystic lesions.
19.7 Summary
1.
Cystic pancreatic tumors and tumorlike lesions represent a wide spectrum of histopathology from purely benign to overly malignant.
2.
The most important diagnostic strategy for accurate characterization of cystic pancreatic tumors is to differentiate non-mucinous cystic tumor from mucinous types of tumors.
3.
The most common cystic pancreatic tumor is intraductal papillary mucinous neoplasms (IPMNs).
4.
The most important diagnostic clue for IPMN is to detect communication between the lesion and pancreatic duct.
5.
For IPMN, the risk of malignancy increases with older age, presence of symptoms, involvement of the main pancreatic duct, dilation of the main pancreatic duct over 10 mm, the presence of mural nodules, and size >3 cm for branch duct type IPMN.
6.
Serous cystic neoplasm (SCN) can be further categorized into 5 different subtypes according to their macroscopic appearances: serous microcystic adenomas, serous oligocystic ill-defined adenoma, von Hippel-Lindau-associated serous cystic neoplasm, solid serous adenoma, and serous cystadenocarcinoma.
7.
SCN tends to have a lobulating outer margin, while mucinous cystic neoplasm has a smooth outer margin.
8.
Solid pseudopapillary neoplasm is the most common solid pancreatic tumor which can mimic cystic pancreatic tumor due to a tendency of cystic degeneration.
19.8 Illustrations: Cystic Tumors of the Pancreas
19.8.1 Schematic Diagrams of Cystic Pancreatic Lesions
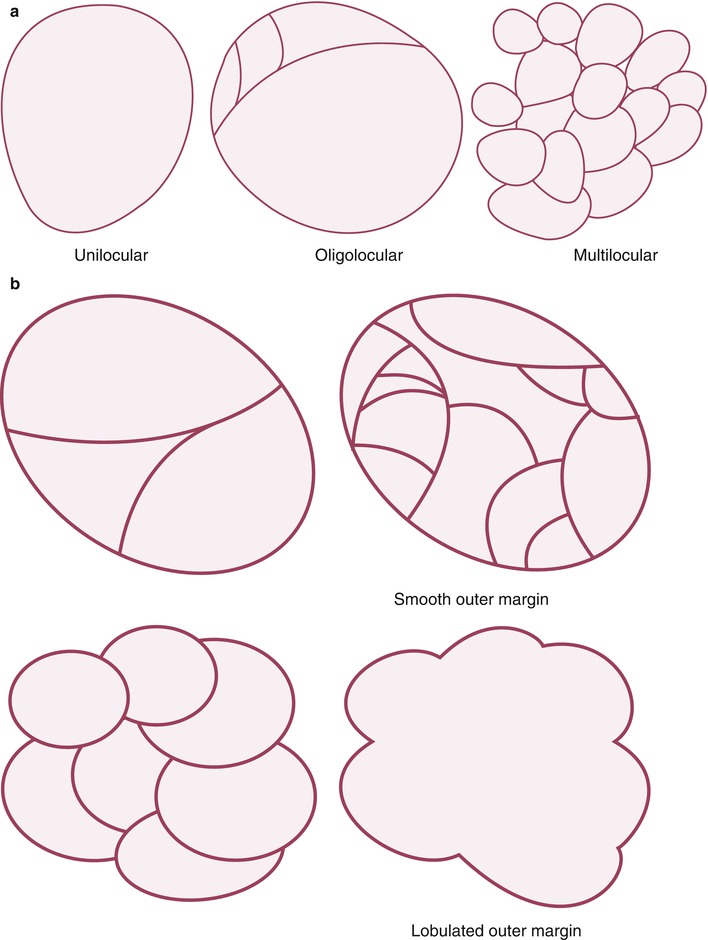
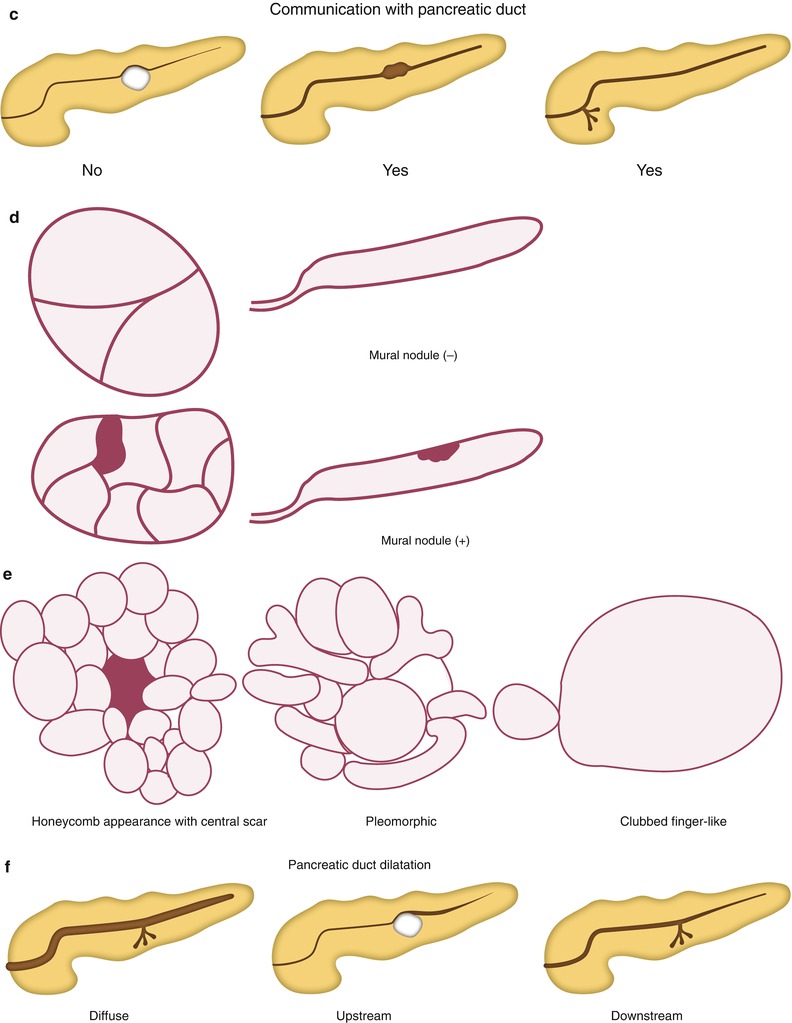
Fig. 19.1
Classification of cystic pancreatic lesions. (a) According to the number of the cyst, cystic lesions can be subdivided by unilocular (one cyst), oligolocular (2–6 cysts), and multilocular (seven or more cysts) lesions. (b–d




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree