Abstract
Maternal infections with the TORCH viruses (toxoplasmosis, rubella, cytomegalovirus [CMV], and herpes simplex) as well as varicella can develop multiple sequelae depending on the timing of infection and variable responses. Common effects include central nervous system abnormalities, growth restriction, and fetal hydrops.
Keywords
ventriculomegaly, intracranial calcifications, intrauterine infection, TORCH infection, fetal hydrops, fetal growth restriction
Cytomegalovirus
- Sonya S. Abdel-Razeq
Introduction
The acronym TORCH refers to a group of organisms associated with congenital infections: t oxoplasmosis, o ther (congenital syphilis and viruses), r ubella, c ytomegalovirus (CMV), and h erpes simplex virus (HSV). The approximate number of cases per year in the United States of these infections is listed in Table 165.1 . In the United States, CMV is the most common fetal infection.
| Infection | Cases/Year in the United States |
|---|---|
| CMV | 40,000 |
| Toxoplasmosis | 400–4000 |
| Herpes simplex | 100 |
| Varicella | 70 |
| Rubella | 11 |
In the United States, CMV, a deoxyribonucleic acid (DNA) herpesvirus, is the most common viral infection of the fetus, affecting nearly 40,000 infants annually. This ubiquitous virus causes a wide variety of clinical manifestations.
Disease
Prevalence and Epidemiology
CMV is the most common cause of in utero infection, with a birth prevalence of 0.5% to 2.5%. Seroprevalence for CMV varies with geographic region, income, and ethnicity and ranges from 40%–83%. Previous exposure to CMV is greater in women of lower socioeconomic status and in developing countries.
Humans are the only known reservoir for human CMV, which is excreted in most bodily fluids of an infected individual. Saliva, urine, cervical secretions, semen, and breast milk are all potential sources for infection.
The fetus may be infected by either a primary or a secondary maternal infection. Vertical transmission is 30% to 50% after primary infection ( Fig. 165.1 ) and less than 1% after secondary infection ( Fig. 165.2 ). Maternal antibodies to CMV do not prevent reactivation of latent disease or reinfection with CMV strains different from that acquired during primary infection. Although maternal immunity reduces maternal-fetal transmission, the severity of congenital CMV may be the same for recurrent or reactivated maternal CMV as for primary infection (see Fig. 165.2 ).

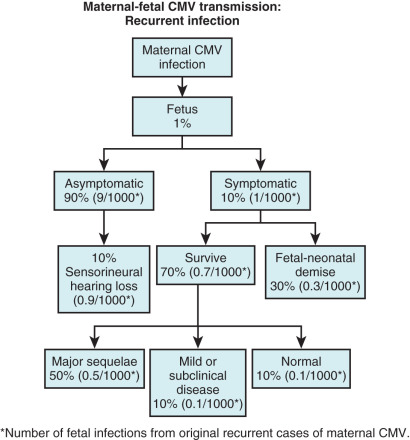
The likelihood of fetal infection increases, and the prevalence of severe congenital infection decreases with advancing gestation. In general, 10% of infected newborns are symptomatic at birth, and an additional 10%–15% of asymptomatic infected newborns have late neurologic sequelae during the first 2 years of life. In the latter group, injury is usually limited to sensorineural hearing loss (see Figs. 165.1 and 165.2 ) but may also include cognitive deficit, seizures, and death. Auditory damage is caused by viral replication in sensory neuroepithelium and a subsequent host immune response. The prognosis among symptomatic infants is poor; survivors generally have serious permanent abnormalities. The severe neurologic complications of CMV are correlated with the presence of hematologic signs, specifically hepatosplenomegaly. Approximately 3% to 5% of neonates acquire CMV in the peripartum period from infected cervical secretions or infected breast milk.
Etiology and Pathophysiology
CMV is a member of the herpesvirus family, and thus demonstrates periods of latency and reactivation with viral shedding. The incubation period after infection is 3 to 12 weeks. The virus is excreted for months to years after infection and may remain latent with excretion recurring if immunosuppression of the host occurs. CMV replicates in the placenta, impairing placental development and resulting in placental insufficiency ( Figs. 165.3 and 165.4 ). The placenta can serve as a reservoir for viral replication before embryo or fetal infection. CMV may result in a first-trimester miscarriage without infecting the fetus.


Manifestations of Disease
Clinical Presentation
In immunocompetent adults, CMV is generally mild—with nonspecific symptoms of fatigue, myalgia, headache, rhinitis, pharyngitis—and self-limited, and may even be clinically asymptomatic in approximately 90% of cases. Conversely, active infection may present as an illness similar in appearance to mononucleosis with symptoms of fever, myalgias, bronchitis, and a flu-like syndrome. In more severe cases, pneumonia and hemolytic anemia may occur. In immunodeficient individuals, CMV may cause pneumonia as well as hepatitis and gastrointestinal symptoms.
Congenital CMV has a similar range from mild disease to severe generalized infection with multiorgan disease and nonimmune hydrops. With severe disease, in utero demise may occur. Most newborns of women with either primary or secondary infection are asymptomatic at birth; however, 10%–15% of these ultimately develop neurodevelopmental damage within the first 3 years of life. The sequelae in neonatal survivors of severe systemic CMV may also include microcephaly, hearing loss, and motor disabilities. The available data suggest that the range of severity of clinical abnormalities in congenitally infected newborns is the same whether the mother has a primary or nonprimary infection. The severity of fetal disease is determined by the timing of infection and the affinity for nervous tissue of various CMV strains. It has not been determined why the fetal brain is more susceptible to CMV than the adult brain.
In multiple gestation, CMV infection may affect one or both twins, regardless of placentation. When both twins are infected, the severity of disease may vary.
Diagnosis.
The diagnosis of clinically suspected maternal CMV infection is based on serology. The new appearance of CMV-specific immunoglobulin (Ig)G is diagnostic of new acute infection. IgM is not a reliable marker for acute infection or timing exposure for several reasons:
- •
IgM may remain positive for over 1 year following acute infection.
- •
IgM may revert to positive from negative in women with reactivation or reinfection with a new viral strain.
- •
It is present in 75%–90% of those with acute infection, and there exists a high false-positive rate for IgM assays performed in commercial, nonreference laboratories.
Despite the availability of avidity testing, the diagnosis of acute infection may remain elusive as intermediate results are difficult to interpret and the appropriate cutoff for low avidity is not universally defined. In the setting of maternal infection and/or findings on ultrasound (US) suggestive of fetal infection (echogenic fetal bowel, cerebral periventricular echogenicities, hepatic calcifications, microcephaly, fetal growth restriction), amniocentesis should be considered to perform polymerase chain reaction (PCR) for CMV DNA in amniotic fluid. Timing of this procedure is critical as test sensitivity is highest after 21 weeks’ gestation and greater than 6 weeks between maternal infection and procedure. This 6-week lag time allows for placental viral replication, transmission to fetus, viral replication in the fetal kidney, and excretion into amniotic fluid. If amniocentesis is performed earlier in gestation or soon after diagnosis of maternal infection, it is reliable evidence of fetal infection if positive, but should be repeated later in gestation if negative.
PCR is the preferred test, as sensitivities of viral isolation from amniotic fluid vary from 45%–80%. The prognostic value of DNA quantification of CMV in amniotic fluid is controversial. Lazzarotto et al. reported that with viral loads less than 10 3 copies/mL, there is a low risk of symptomatic neonatal infection. Benoist et al. did not find a correlation between viral load and the likelihood of symptomatic neonatal CMV.
Imaging Technique and Findings
Ultrasound.
Between 16 and 36 weeks’ gestation, women with primary CMV infection and newborns with symptomatic CMV have significantly thicker placentas than control women without CMV. At 20 weeks’ gestation, placental thickness in control pregnancies without CMV was 20 mm compared with 40 mm ( Fig. 165.5 ) in CMV-infected pregnancies. Some of the US findings associated with CMV (e.g., intrauterine growth restriction [IUGR]) may be caused by placental dysfunction ( Fig. 165.6 ) rather than from direct viral infection. This placental dysfunction may partly explain why CMV DNA load in amniotic fluid does not correlate with the severity of abnormalities associated with congenital CMV infection visualized on US.


Of congenital infections, CMV is the major cause of destructive cerebral lesions ( Table 165.2 and Fig. 165.7 ). Brain damage is caused by a direct cytopathic effect and an immune inflammatory response to CMV. Because the fetal brain is a focus of all congenital infectious evaluations, transvaginal US should always be attempted when the vertex is in a suitable position to detect subtle abnormalities.
|

Neurons form between 8 and 20 weeks’ gestation; migration of neuronal cells to the cerebral cortex continues until 24 to 26 weeks’ gestation. Both neuronal loss and a disturbance in neuronal migration may play a role in the development of microcephaly associated with CMV infection.
A periventricular halo (see Fig. 165.7E ) is indicative of white matter injury and is detected before brain calcifications. The periventricular halo is initially noncontinuous and may be appreciated only with transvaginal US. As white matter damage progresses, the halo coalesces and is detectable with transabdominal US. The duration and severity of the cerebral infection determine whether ventriculomegaly is present at the time of the periventricular halo. Focal symmetric periventricular cysts generally cannot be detected until the third trimester.
Cerebral calcifications associated with CMV are generally periventricular in location. Calcifications associated with toxoplasmosis are scattered throughout the brain.
Lenticulostriate vasculitis results in the deposit of amorphous material in the walls of the vessels within the basal ganglia and thalamus. Although the vessels are echogenic, they are not occluded. Resolution of the lesions may occur. However, with severe infections, the vessel walls eventually may become calcified. These linear echogenicities have also been found with other infections (rubella, syphilis), chromosomal abnormalities (trisomies 21 and 18), and prematurity.
Anomalies of the cerebellum (i.e., vermian hypoplasia) suggest that the CMV infection occurred before 18 weeks’ gestation, when cerebellar formation is complete. Lissencephaly is a disturbance of neuronal migration between 3 and 5 months’ gestation. US diagnosis of lissencephaly can be made at around 28 weeks’ gestation. The presence of cerebellar hypoplasia (see Fig. 165.7F ) in association with lissencephaly should suggest a diagnosis of congenital CMV infection.
In one series of 73 in utero CMV infections, 34 of 73 (52%) had US findings; 27 of 34 (79%) infections involved the central nervous system (CNS). There were isolated extracerebral US abnormalities in 11 (32.4%) infections. Simon-Bouy et al. prospectively evaluated 682 cases of fetal echogenic bowel ( Fig. 165.8 ). CMV was found in 15 (2.2%) fetuses. In 11 of 15 fetuses with CMV, echogenic bowel was the only US abnormality detected. Echogenic bowel or bowel dilatation is caused by viral enterocolitis.

Isolated hepatic echogenic foci are infrequently associated with infection and are usually caused by a vascular accident. Multiple hepatic calcifications secondary to hepatitis are more likely associated with congenital infection. Hepatosplenomegaly is common with severe symptomatic congenital CMV ( Fig. 165.9 ).

Occasionally, hydrops ( Fig. 165.10 ; see Fig. 165.5 ) secondary to CMV infection is detectable by 16 weeks’ gestation. This early manifestation of hydrops is secondary to hepatic dysfunction, liver congestion, and subsequent anemia. Myocarditis can also play a role in the development of hydrops. Isolated fetal ascites is caused by hepatic dysfunction. When the liver recovers, the ascites resolves. IUGR is present in 50% of symptomatic infants infected with CMV; the prematurity rate is 34% in this subgroup. Renal involvement may result in temporary or persistent oligohydramnios and, occasionally, polyhydramnios. Calcifications of the retina secondary to chorioretinitis have been detected on third-trimester US examinations of fetuses with CMV.

The reported US markers associated with congenital CMV are outlined in Table 165.2 . These findings may be transient or become manifest only late in pregnancy, requiring serial US examinations to be detected. Although the possible US markers are numerous, they are not specific.
The US detection rate of congenital CMV is difficult to assess because of the variables listed in Table 165.3 . Crino combined data from three studies to obtain a 30% sensitivity, 98% specificity, 90% positive predictive value, and 70% negative predictive value for the detection of congenital CMV with US. In one study, 23 of 154 congenitally infected fetuses (14.9%) had identifiable US findings. The positive predictive value of US in detecting congenital infections was 35.3% for all fetuses of mothers with primary CMV and 78.3% for fetuses with documented infection. In two series of 62 infected fetuses with US abnormalities, approximately half of abnormalities were detected between 18 and 22 weeks’ gestation. The remaining 50% of the detectable anomalies associated with congenital CMV were identified after a gestational age of 22 weeks. A normal second-trimester US examination does not exclude an abnormal US examination at a later gestational age or the birth of an infant with significant CMV sequelae.
|
Prognosis is based mainly on the presence of fetal brain abnormalities. However, all detectable brain lesions do not have the same prognostic significance. Even ventriculomegaly, considered one of the most ominous US findings, does not invariably result in long-term sequelae. When US abnormalities are detected, neonates may still escape significant morbidity. Nonspecific isolated US findings (i.e., echogenic bowel) are not associated with a poor outcome.
Magnetic Resonance Imaging.
MRI may provide additional information in the assessment of a fetus suspected to have congenital CMV. Even with a normal fetal anatomic survey, MRI has detected subtle brain abnormalities, increasing the positive predictive value of prenatal assessment. Although brain anomalies associated with CMV infection may be detected on MRI before 24 weeks’ gestation, the results can be misleading. Repeat MRI in the third trimester is required to better elucidate the prognostic reliability of the earlier MRI examination or to monitor previously diagnosed brain abnormalities.
The type of brain abnormalities detected on MRI helps to approximate the time of fetal infection. Lissencephaly suggests a brain injury before 16 to 18 weeks’ gestation, whereas polymicrogyria is associated with an injury between 18 and 24 weeks’ gestation. The development of a normal gyral pattern indicates the fetus was not infected until the third trimester. Despite the possibility of detecting abnormalities, MRI use remains controversial as normal brain imaging does not necessarily predict normal neurodevelopmental outcomes.
Intracranial and intrahepatic calcifications.
Differential Diagnosis From Imaging Findings
Although there are numerous US signs associated with CMV, none are specific. With documented maternal CMV, the US findings listed in Table 165.2 are strongly associated with congenital CMV. The differential diagnosis depends on the specific US findings associated with a particular case of congenital CMV. In general, the US findings listed in Table 165.2 can be found with other congenital infections.
- 1.
Echogenic bowel as an isolated finding may be a physiologic variation of normal or be caused by cystic fibrosis, aneuploidy, bowel obstruction, or congenital CMV.
- 2.
CMV is only one of a long list of etiologies for nonimmune hydrops. A detailed maternal history, an extended fetal anatomic survey, maternal blood work, and amniocentesis help to narrow the differential diagnosis.
Synopsis of Treatment Options
Prenatal
The use of antiviral medications may be indicated for treatment of maternal end-organ CMV disease, but have not been shown to decrease vertical transmission. However, the use of antivirals in symptomatic neonates may potentially decrease neonatal morbidity and mortality. Hyperimmunoglobulin (HIG) therapy has been reported to cause regression of cranial and abdominal US sequelae associated with CMV. The first randomized trial to address the use of CMV HIG (the CHIP study) was recently completed in Italy and did not show a significant reduction in congenital infection. This study randomly assigned 124 women with primary CMV infection to either CMV HIG or placebo. There was no difference in the viral characteristics of the infected neonates and similar viral loads in amniotic fluid and neonatal urine were detected among the groups. Interestingly, the HIG arm was notable for increased risk of adverse outcome, specifically preterm delivery, preeclampsia, fetal growth restriction. The risk was not statistically significant. There is currently one trial in progress addressing HIG use.
Postnatal
Treatment is generally not necessary or recommended for healthy children and adults. Newborns are treated if they are symptomatic. Antiviral medications (ganciclovir, cidofovir, and foscarnet) are used to prevent viral division. However, these drugs are not curative. Two CMV vaccines have been evaluated, but a reliable vaccine is not yet available.
- •
CMV is the most common cause of in utero infection.
- •
US signs associated with CMV are multiple and nonspecific.
- •
As the number of US findings increases, the likelihood of CMV as the cause also increases.
- •
The sensitivity of US examination in detecting fetal CMV is 30%; only half of the US signs are detected before 22 weeks’ gestation.
- •
A normal second-trimester US examination does not exclude a severely affected fetus.
- •
A diagnostic amniocentesis should not be performed until 6 weeks after maternal exposure and after 21 weeks’ gestation.
- •
An abnormal prenatal US examination, a low neonatal platelet count, and microcephaly have been identified as the most predictive markers of adverse outcome after an in utero CMV infection.
Acknowledgment
The authors are indebted to Lyndon M. Hill, MD, for his work on this chapter in the first edition, and for generously contributing the images.
Suggested Readings
Rubella
- Sarah N. Cross
Introduction
Rubella, which is also called German measles , was first described in the 1750s. In the 1940s an association between congenital cataracts and maternal rubella was noted during an epidemic in Australia. Subsequently, congenital rubella syndrome (CRS) was identified. There was a worldwide rubella pandemic between 1962 and 1965; in the United States between 1964 and 1965 there were an estimated 12.5 million cases of rubella, 11,250 fetal deaths, 2100 neonatal deaths, and 20,000 infants born with CRS. In 1969 a vaccine was introduced in the United States that substantially decreased the incidence of rubella infection.
Disorder
Definition
Congenital rubella infection (CRI) refers to all pregnancy outcomes associated with intrauterine rubella infection including miscarriage, fetal demise, congenital malformations, as well as asymptomatic infection. CRS refers to a variable constellation of birth defects such as hearing impairment, congenital heart defects, cataracts/glaucoma, and retinopathy. To meet criteria for CRS, there has to be more than one identifiable birth defect.
Prevalence and Epidemiology
Once rubella became a nationally notifiable disease in 1966, accurate estimates of the number of cases in the United States was possible. In 1969 just before the introduction of the vaccine, the reported incidence of rubella was 57,686 cases. By 1983 fewer than 0.5 cases per 100,000 people were reported.
In 2004 rubella was officially declared eliminated from the United States, and in 2015 rubella was officially declared eliminated from the Americas. However, cases have been seen in the United States, potentially because of a drop-off in vaccination; in 2013 there were nine cases of rubella and one case of CRS reported in the United States.
Worldwide, the incidence of rubella correlates with economic development. By 2002 rubella immunization existed in 100% of industrialized nations, 71% of countries with transitioning economies, and 48% of developing countries. Rubella vaccine was introduced nationwide in 140 countries by the end of 2014, up from 85 countries in 1996.
Etiology and Pathophysiology
Rubella virus is a single stranded ribonucleic acid virus that belongs to the Rubivirus genus and the Togavirus family. Humans are the only known natural host. The virus replicates primarily in the nasopharynx, and primary infection occurs by respiratory transmission. Viremia begins 5 to 7 days postinfection. Rubella infection persists for an average of 14 days (range 12–23 days) with a prodrome of low-grade fever and lymphadenopathy in the second week, followed by macropapular rash 14–17 days postexposure. Infectivity is possible from 1–2 weeks before the onset of symptoms to 14 days after the rash has resolved. Infection usually confers immunity; however, reinfection can occur.
During maternal viremia, maternal-fetal transmission can occur via hematogenous spread. After infecting the placenta, the virus spreads through the fetal vascular system. Rubella virus is thought to exert its teratogenic effects via vasculitis or apoptosis.
Manifestations of Disease
Clinical Presentation
CRS refers to a variable constellation of birth defects often including hearing impairment, congenital heart defects, cataracts/glaucoma, and retinopathy, as well as fetal growth restriction, hypotonia, developmental delay, and seizures. Miller et al. followed 1016 cases with confirmed maternal rubella infection. The frequency of congenital infection varied with gestational age; it occurred in more than 80% of women infected in the first trimester, and 54% infected between 13 and 14 weeks, decreasing to 25% by the end of the second trimester. Improved fetal immune response and transfer of maternal antibodies across the placenta after 16 weeks are thought to make congenital damage less severe after 16 weeks.
Imaging Technique and Findings
Ultrasound.
The most common US findings associated with rubella infection include cardiac defects (atrial and ventricular septal defects, pulmonary artery hypoplasia, and after birth, patent ductus arteriosus), ocular anomalies (cataracts and microphthalmia), microcephaly, hepatomegaly, splenomegaly, and growth restriction. Less common anomalies include peripheral pulmonic stenosis, glaucoma, meningocele, renal disorders, hypospadias, meconium peritonitis, and hyperechoic bowel as well as intracranial calcifications and subependymal pseudocysts.
In pregnancies suspected to be complicated by rubella infection, a detailed fetal US should be performed, with special attention to intracranial, ophthalmic, and cardiac structures. If necessary, transvaginal US should be performed for optimal intracranial imaging. A fetal echocardiogram should also be performed.
Cardiac defects (atrial and ventricular septal defects, pulmonary stenosis).
Ocular anomalies (cataracts and microphthalmia).
Microcephaly.
Hepatomegaly.
Splenomegaly.
Growth restriction.
Magnetic Resonance Imaging.
The CNS findings make MRI an ideal modality to assess the fetus in the late second or third trimester. MRI may be more sensitive than US for minor atrophic changes and white matter lesions.
Differential Diagnosis From Imaging Findings
Several congenital infections have overlapping findings, such as cataracts and hepatosplenomegaly. Therefore all the TORCH infections (e.g., t oxoplasmosis, o ther [syphilis, varicella], r ubella, c ytomegalovirus, and h erpes) should be considered in the differential diagnosis. In addition, several syndromes have similar findings, such as Smith-Lemli-Opitz syndrome, an autosomal recessive condition caused by 7-dehydrocholesterol reductase deficiency that includes growth restriction and congenital heart disease. With an estimated incidence of 1 in 20,000, Smith-Lemli-Opitz syndrome is more common than congenital rubella.
Evaluation of maternal rubella immunization status, evaluation for other TORCH infections as well as carrier testing for Smith-Lemli-Opitz syndrome can be performed to help determine the correct diagnosis.
Synopsis of Treatment Options
Prenatal
The most important management strategy for CRS is prevention, as there is no effective treatment. Females of childbearing age should be immunized against rubella if nonimmune. Women planning pregnancy should have their immunization status checked preconception and vaccinated as necessary. Pregnant women should also have their immunization status checked. Nonimmune women should be counseled on avoidance of individuals with suspected rubella and then be vaccinated postpartum. Importantly, the vaccine is not administered during pregnancy, as the live attenuated vaccine can be transmitted to the placenta, although there is no evidence of CRS occurring after immunization.
Women suspected of having a pregnancy affected by rubella infection should be referred to a physician with expertise in the area. Prenatal consultations depending on organ system involvement are recommended. Delivery at a tertiary care center is recommended.
Postnatal
Postnatal management involves vaccination for prevention in unaffected infants. The Centers for Disease Control recommends two doses of the measles-mumps-rubella vaccine; the first dose at 12–15 months and the second dose at 4–6 years of age.
Affected infants require multidisciplinary individualized supportive therapies depending on disease status and organ system involvement.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree