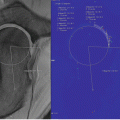
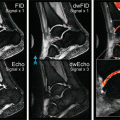
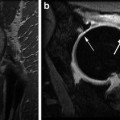
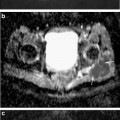
Young-Jo Kim and Tallal Charles Mamisch (eds.)Hip Magnetic Resonance Imaging201410.1007/978-1-4614-1668-5_3
© Springer Science+Business Media, LLC 2014
3. Delayed Gadolinium-Enhanced MRI of Cartilage
(1)
Department of Radiology, Beth Israel Deaconess Medical Center, 4 Blackfan Circle, Boston, MA, USA
Abstract
Delayed gadolinium-enhanced MRI of cartilage (dGEMRIC) was first described in 1996 as a nondestructive technique for quantitative measurements of glycosaminoglycan (GAG) concentration in articular cartilage samples [1]. Its translation to clinical platforms as a means of interrogating the molecular aspects of cartilage was demonstrated in 1997 with illustrative examples of apparent molecular degeneration in cartilage that was grossly intact morphologically [2]. Since then, over 150 reports of dGEMRIC involving in vitro and clinical studies have been published. These reports demonstrate the potential for molecular imaging techniques to illustrate new paradigms in the understanding of cartilage disease. However, along with the expanded applications come questions regarding appropriate protocols, possible sources of measurement and interpretation errors, and insight into directions for future study. This chapter describes the theoretical basis of dGEMRIC, protocols, pitfalls, and opportunities, focusing on the clinical aspects. Further details can be found in several reviews [3–7].
Delayed gadolinium-enhanced MRI of cartilage (dGEMRIC) was first described in 1996 as a nondestructive technique for quantitative measurements of glycosaminoglycan (GAG) concentration in articular cartilage samples [1]. Its translation to clinical platforms as a means of interrogating the molecular aspects of cartilage was demonstrated in 1997 with illustrative examples of apparent molecular degeneration in cartilage that was grossly intact morphologically [2]. Since then, over 150 reports of dGEMRIC involving in vitro and clinical studies have been published. These reports demonstrate the potential for molecular imaging techniques to illustrate new paradigms in the understanding of cartilage disease. However, along with the expanded applications come questions regarding appropriate protocols, possible sources of measurement and interpretation errors, and insight into directions for future study. This chapter describes the theoretical basis of dGEMRIC, protocols, pitfalls, and opportunities, focusing on the clinical aspects. Further details can be found in several reviews [3–7].
Biophysical Basis for dGEMRIC
dGEMRIC, like most biochemical methods for measuring cartilage GAG including radiotracer, histology with cationic dyes, and biochemical assays, is based on biophysical principles elucidated by Maroudas almost 40 years ago [8]. These principles rely on the fact that GAG is the source of the majority of the fixed charge on the cartilage extracellular matrix, due to the abundant carboxyl and sulfate groups on the GAG molecules. Mobile ions will distribute in tissue according to the GAG (negative fixed charge) concentration. The associated theories can be used to calculate the concentration of GAG based on the concentration of the mobile ion in the tissue. In dGEMRIC the mobile ion is the MRI contrast agent Gd-DTPA2− (Gadopentetate Dimeglumine, Bayer Health Care).
If Gd-DTPA2− is allowed time to equilibrate in tissue, theoretically the concentration of Gd-DTPA2− can be determined from a measurement of the MRI parameter T1 in the presence of Gd-DTPA2− (T1(Gd)), and the concentration of GAG can then be calculated from the determined concentration of the mobile Gd-DTPA2− ion. In practice, the conditions for quantitation of absolute GAG are not met in clinical studies due to the contrast agent not being in steady state with the cartilage, and the relative distribution of T1(Gd) is utilized as a metric for the relative distribution of cartilage GAG, as described below.
Protocol Considerations and Pitfalls
In order to determine the GAG concentration in cartilage from the concentration of Gd-DTPA2−, Gd−DTPA2− needs to be added to the system, and it needs time to penetrate the cartilage (which may take up to several hours in the avascular tissue). T1 measurements are then needed in order to assess the distribution of Gd-DTPA2− concentration. There are several protocol considerations with respect to these steps:
T1 pre-contrast
Theoretically, calculation of Gd-DTPA2− requires knowledge of T1 both before and after contrast agent administration. However, under some conditions the variation of T1 without contrast across the tissue in an individual, or between individuals, may be small enough relative to the changes induced by the contrast agent that it is possible to utilize the T1 post-contrast alone as a metric of Gd-DTPA2− concentration. This has generally been found to be the case in dGEMRIC studies of native cartilage tissue of the knee, hip, and finger [9–12].
Administration of Gd-DTPA2−\n
In clinical studies, the Gd-DTPA2− is administered either by intra-articular or intravenous injection. Early studies demonstrated that in the thick cartilage of the patella of the knee, the kinetics of penetration of Gd-DTPA2− was faster with intravenous administration due to its penetration from both the synovial and bone surfaces [2]. However, this may be less of an issue for the thinner cartilages of other aspects of the knee or of the hip.
An advantage of the intra-articular injection is the improved delineation of cartilage in addition to the dGEMRIC effect [13, 14]. However, intravenous is generally easier to implement and thus has been utilized in the majority of dGEMRIC studies to date of the knee, although a number of recent studies have utilized intra-articular injections for dGEMRIC of the hip [13–16].
Dose
Increasing dosage of Gd-DTPA2− results in easier delineation of differences in GAG, as well as lower T1(Gd) and hence faster T1(Gd) measurement. Offsetting this is the concern for the safety issues of contrast agent at higher doses [17]. The majority of dGEMRIC studies have been done with “double dose” Gd-DTPA2− (0.2 mmol/kg), although utility of single dose has also been illustrated [18].
Another consideration with dosing is that, since adipose tissue does not take up Gd-DTPA2− as much as lean mass, dosing by weight results in individuals with higher BMI given an effectively larger dose of Gd-DTPA2− [19]. Therefore, in studies including subjects with a large range of BMI, one can either adjust the dose, or “correct” the dGEMRIC values post-acquisition [19]. The BMI effect will not be an issue for longitudinal studies of individuals where BMI does not change during the study period. Alternatively, studies of relative dGEMRIC values within the knee at one time or across time will demonstrate effects apparent in localized regions of the knee even if BMI changes might be of concern [20].
Delay Period
Once injected, the Gd-DTPA2− needs time to penetrate into the cartilage. Early studies demonstrated that 30–90 min were effective for penetration into hip cartilage, and 2–3 h into all the cartilages of the knee [21]. In particular, thicker cartilage would require a longer time for contrast agent penetration, and incomplete penetration of contrast in thick cartilage may result in higher T1(Gd) than would be obtained after longer equilibration times [22]. Therefore, early loss of GAG in thick cartilage may be underrepresented by this technique. Potentially offsetting the effect of longer penetration time in thick cartilage and overestimation of the GAG concentration is the effect of faster penetration kinetics of contrast agent into degraded tissue [23].
The faster penetration of Gd-DTPA2− into degraded cartilage also implies that earlier time points after contrast agent injection will result in a larger observed effect difference between healthy and diseased tissue [24] as the Gd-DTPA2− will enter degraded tissue faster and will go to a higher concentration [25], and then wash out faster [26].
In interpreting clinical studies and clinical trials, the time after injection should be taken into account and consistency of this time noted. Most hip studies have been done with a delay time of about 30 min after injection; most knee studies have been done after 90 min.
Joint Motion
The initial clinical studies demonstrated that some form of motion of the joint is necessary for consistent penetration of the Gd-DTPA2− into cartilage [21]. The protocols for this motion have varied from walking 10 min, to climbing stairs, to passive motion of the joint. No definitive study has yet been undertaken to define whether one type of motion is better than another.
T1 Imaging
Although T1 weighted images might demonstrate areas depleted of cartilage [2], quantitation of T1(Gd) allows for an absolute index for direct comparison across people and time points.
Calculated images of T1 can be obtained through some form of inversion recovery or saturation recovery pulse sequence. The most straightforward, although also the most time-consuming, are standard 2D inversion recovery pulse sequences. These are generally limited by the number of sections that can be imaged in a reasonable imaging time, and therefore are appropriate if a given section of the joint is of interest and can be localized to easily from scout scans.
More generally, three-dimensional pulse sequences allow for coverage of the joint and post-processing of the region of interest. This is particularly of value with the relatively spherical geometry of the hip joint. A number of studies have validated 3D T1 imaging compared to 2D sequences [27–31], and low versus high resolution imaging [32].
After the imaging data are obtained, calculated T1 maps are made through a number of software packages available. In general the data are reported as mean T1(Gd) from a region of interest.
Field Strength
Both 1.5T and 3T are commonly used for musculoskeletal imaging applications. While generally 3T would have advantages in terms of achievable resolution, the higher T1 values at 3T result in a longer imaging time for dGEMRIC at that field strength. In addition, B1 inhomogeneity, which can be a factor in the accuracy of T1 measurements, is more problematic at 3T than at 1.5T. Correction schemes for B1 inhomogeneity have recently been proposed [36–38]. Pilot studies have also recently been reported at 7 T [39].
Combination with Other Pulse Sequences
A number of parameters have been proposed for interrogating the molecular aspects of cartilage, as discussed in other chapters in this book. There have been several proposals to utilize combined information from several parameters, which raises the issue of whether they can be measured in one scan session.
Since T2 is much more sensitive to tissue macromolecules than to Gd-DTPA2−, T2 is not significantly impacted by Gd-DTPA2−, and it is possible to measure both T2 and dGEMRIC after administration of Gd-DTPA2− [40, 41]. This is not true for T1rho, which would be strongly affected by the concentration of Gd-DTPA2−. Therefore, combined measures of T1rho and dGEMRIC would require imaging both before and after contrast agent administration.
The accuracy of cartilage thickness or volume measurements in the presence of Gd-DTPA2− has been investigated for knee cartilage. Baseline measurements were found to be comparable with or without the contrast agent in the tissue [42]; however, a later study found that changes in thickness over time yielded disparate results depending on whether the measurements were made in the presence of Gd-DTPA2− [43]. Improved accuracy may be a matter of optimizing the segmentation routines for post-Gd-DTPA2− tissue contrast, and/or improving inherent errors in the volume measurement themselves.
Reproducibility of T1 Measurements
Reproducibility of studies needs to be interpreted in the context of the level of changes that need to be evaluated for a given study. Measurements of reproducibility of dGEMRIC are also complicated by the kinetics of the contrast agent washing in and out of cartilage. Due to the contrast agent administration, two complete studies immediately following one another are also not appropriate. Therefore, many of the reproducibility studies have been done with scans several weeks apart, and the results are a combination of actual measurement reproducibility and variation in the natural levels of cartilage molecular composition over these short time frames.
With this in mind, the reproducibility data can be evaluated. Early studies showed a reproducibility of about 15 % in the knee [21]. This has been improved with faster pulse sequences and better analysis routines. In the knee, the reproducibility has been shown to be between 5 and 8 % depending on the size of the regions of interest [44], while in the hip similar values were obtained (3.7–6.8 %) [45]. Interobserver variability in drawing standardized regions of interest was found to be better than 3 % in the different compartments of the knee [46]. Image registration was found to improve the reproducibility in the knee with ICC ranging from 0.85 to 0.9 [47, 48].
Since many of the clinically observed variations within a joint, between people, or changes over time are on the order of 20 % and higher (see sections below), the reported reproducibility values are sufficient for many applications.
Validation Studies
The theories developed by Maroudas [8] assume that the cartilage is in equilibration with a large “bath” (surrounding source) of the mobile ion, in this case Gd-DTPA2−. These conditions are relatively easily achieved in vitro by placing the cartilage sample in a solution containing Gd-DTPA2− for several hours, and a number of bench studies have validated the concentration of GAG as determined by dGEMRIC against biochemical and histological metrics [1, 49].
In clinical studies, the conditions of equilibrium and infinite bath surrounding the tissue do not hold. Therefore, the relative distribution of GAG within a joint, and between individuals, is inferred from the T1(Gd) maps. These distributions measured in vivo have compared well to histology from samples obtained after the imaging from total knee replacement surgeries [49]. Another means of validating the clinical dGEMRIC studies is to compare T1(Gd) images obtained with Gd-DTPA2− to those obtained with a nonionic contrast agent. A small study initially demonstrated that the T1 distribution after administration of a nonionic contrast agent was uniform, compared with the “lesions” seen in the presence of Gd-DTPA2− [2].
Opportunities
Within the context of the protocol and interpretation issues described above, the ability to distinguish molecular characteristics of morphologically intact tissue has the potential to greatly enhance our understanding of cartilage physiology and disease and impact therapeutic planning and evaluation. The majority of dGEMRIC studies have been in the knee and hip, although there are a number of reports of dGEMRIC applied to the finger joints [12, 50–52] and ankle [53]. dGEMRIC protocols have also been applied in the meniscus [54–56] and intervertebral discs [57, 58]; however, the considerations of transport are much more of a concern in these thick tissues, and the interpretation of the studies is likely to be different than those in the articular cartilages.
Applications of dGEMRIC in the hip will be described in detail in a later chapter; here we briefly present evidence for general types of opportunities that have been demonstrated in the hip as well as other joints that might be applicable to the hip in future studies.
Cartilage Physiology and Pathophysiology
While imaging studies tend to focus on disease, a better understanding of physiology might lead to lifestyle changes or interventions that might prevent degeneration of the joint. For example, adherence to an exercise regimen was shown to increase the dGEMRIC Index [48, 59, 60]. Similarly, in obese subjects, quadriceps strength was shown to correlate positively with dGEMRIC [61], as did weight loss [20]. The implication of biomechanics in alterations in molecular metrics is further strengthened by the observation that knee malalignment has been shown to correlate to medial/lateral ratios of the dGEMRIC Index [62]. Similarly, different types of femoroacetabular impingement had different distributions of dGEMRIC across the hip [63]. The observations regarding the impact of biomechanics may provide a window for therapeutics such as modifying the mechanical tissue conditions.
Pre-radiographic Disease
The most straightforward application of dGEMRIC or any molecular imaging technique is simply to demonstrate and follow “lesions” in otherwise apparently normal-appearing cartilage. Detection and monitoring of these lesions demonstrate a number of paradigm-changing concepts in the evaluation of cartilage physiology and pathophysiology. In particular, molecular scans such as dGEMRIC have demonstrated “lesions” or generally low values in radiographically normal compartments [51, 62, 64], which has implications for the enrollment of such individuals as “controls” in natural history or intervention trials for cartilage disease.
The prior reliance on radiographic abnormalities which only progress has led to the paradigm that arthritis only progresses with worsening disease. The observation of molecular level lesions in radiographically normal or stable states also allows for monitoring of regression as well as progression of disease or injury. In one case report, a posterior cruciate ligament injury was found to result in a dGEMRIC decrease over the first month but then a return to baseline after several months of rehabilitation [65].
Prediction of Disease Progression, Regression, or Success of Intervention
A determination of the molecular status of cartilage might enable one to predict whether the joint is progressing towards worse disease, improving in status, or whether the cartilage is in sufficiently good state such that an intervention can be effective.
One study showed that low dGEMRIC values preceded radiographic OA [66], and another showed that decreasing dGEMRIC in chronic rheumatoid arthritis despite treatment, leading to joint replacement [67]. Similarly several studies have shown the negative impact of ACL injuries, exacerbated by meniscal injuries [68, 69]. Conversely, there are examples of studies demonstrating protective effects, such as muscle strength in knees with meniscal injuries [70




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree