Introduction
It is, perhaps, unfortunate that the myocytes that generate and dissipate the cardiac impulse throughout the myocardial body are known as the cardiac conduction tissues, because all myocytes within the heart possess the capacity to conduct. The alternative title, the “specialized tissues,” is equally unfortunate, because from a developmental stance, as we will show, the so-called conducting tissues, in particular the nodal components, share important features with the myocytes of the primary heart tube. It is their so-called “working” partners that show more evidence of developmental specialization. In this chapter, therefore, we will describe the processes whereby the initial heart tube grows by addition of myocardium at both its venous and arterial pole, how it loops, and how eventually it becomes converted into the four-chambered organ. In this definitive structure, it is the sinus node that generates the cardiac impulse, this being propagated within the atrial musculature, delayed in the atrioventricular node, and then conducted rapidly to the ventricular myocardium through the atrioventricular bundle, its branches, and the ventricular ramifications known as Purkinje fibers. We will also discuss the fate of the more widespread areas of primary myocardium found in the developing heart because it is almost certainly the remnants of these areas in the postnatal heart that are the substrates for many cardiac arrhythmias. In contrast, with regard to perhaps the most important arrhythmia in the ageing population, namely atrial fibrillation, we will show how the pulmonary myocardial sleeves, known now to be the substrate for many forms of atrial fibrillation, are derived from so-called working rather than specialized conducting tissues.
The Basic Cardiac Building Plan
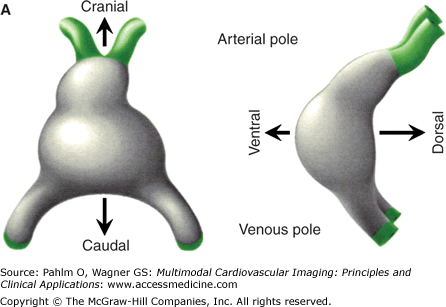
The overall structure of the developing heart and the basic arrangement of the definitive heart are preserved across the vertebrates, from fish to man. When first seen during development, the heart is laid down as a linear myocardial tube, with venous and arterial poles (Fig. 17–1A). Conduction through this linear tube begins at the venous pole and proceeds toward the arterial pole. With ongoing development, cavities balloon from the tube, to which additional material is rapidly added at both poles. In the most basic plan, the myocardium that will form the atrial chambers balloons from the primary tube into the dorsal direction, whereas the myocardium of the developing ventricles balloons in ventral fashion (Fig. 17–1B). Concomitant with the ballooning of these pouches from the linear, or primary, tube, it becomes possible to record an electrocardiogram.1 At these early stages, it is not possible to recognize any “conduction tissues” when assessing the cardiac structure histologically. It is possible, however, to distinguish the characteristics of the myocytes in the different parts of the developing heart according to their molecular signatures. Thus, the myocytes of the primary heart tube do not express the connexin proteins responsible for the formation of fast-conducting gap junctions.2 As a consequence, at this early stage, the blood is propelled through the developing heart in a sluggish, peristaltic fashion. In contrast, the myocytes forming the walls of the developing atrial and ventricular chambers express the molecules that ensure rapid conduction and synchronous contraction, such as the gap-junctional proteins connexin 40 and 43.3 This myocardium making up the walls of the developing chambers is called working, or secondary, myocardium. It is possible to distinguish subtypes within the working myocardium.4 The myocytes making up the atrial working myocardium conduct faster and express the rapidly contracting atrial myosin isoform, whereas the myocytes within the developing ventricles express the slow myosin isoform,5 which is also expressed in slow-twitch skeletal muscle. Whereas myocytes within both the atrial and ventricular working myocardial walls express atrial natriuretic factor, the atrial myocytes that are added via the so-called dorsal mesocardium, the area that will eventually form the myocardial sleeves of the pulmonary veins, do not express this gene (Fig. 17–2).
Figure 17–1.
Morphogenesis of the early heart tube in mammals. A. The prototypic linear heart tube as seen in ventral (left) and right lateral (right) views. B. The prototypic looping and chamber-forming heart tube in similar views. The primary myocardium is gray, the myocardium of the atrial chambers ballooning dorsally in parallel at the venous pole is blue, and the myocardium of the ventricular chambers growing ventrally in sequence along the heart tube is red. At the linear tube stage in human, there is no secondary myocardium present. The chamber myocardium is first seen locally at the stage of looping and does not involve the entire circumference of the tube. Note that the primary myocardium of the venous pole, the atrioventricular canal (AVC), and the outflow tract (OFT) is continuous, with no formation of rings.
Figure 17–2.
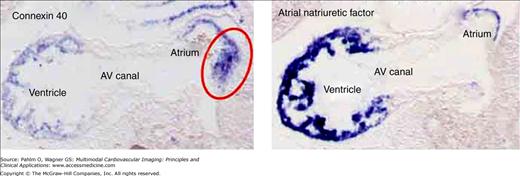
The domains of the atrial musculature are distinguished based on their expression of the different molecular markers. In the left panel, we show of the pattern of expression of mRNA for the gap-junctional protein connexin 40, whereas the right panel shows the pattern of expression of mRNA for atrial natriuretic factor (ANF). Both sections are taken sagittally through the mouse embryonic heart at embryonic day 9½. At the dorsal aspect of the atrium, there is a special part of the muscle, which is positive only for connexin 40 (red circle in left panel). We called this part the mediastinal myocardium. Note the absence of both connexin 40 and ANF in the musculature bordering the atrioventricular (AV) canal.
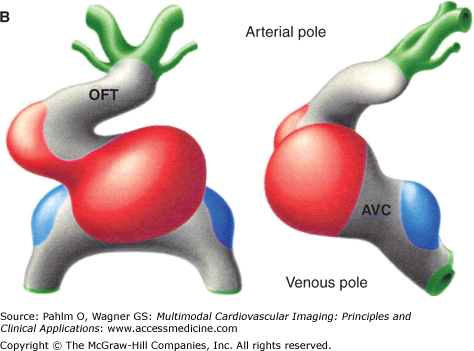
We now know that, in the mammalian heart, the initial component of the linear heart tube forms little more than the embryonic left ventricle and atrioventricular canal. Recent evidence from the mouse heart shows that, from the stance of lineage, the ventricular septum is derived from this embryonic left ventricle, whereas the entire mural component of the left ventricle is derived from the atrioventricular canal.6 Additional material remains to be added to the developing heart at both the venous and arterial poles from the so-called second heart field, although it is debated whether this is derived from a separate field or rather from ongoing temporal migrations of cells from a single source.7,8 Regardless, the end result is that the heart tube elongates and bends concomitant with the addition of the new material at its venous and arterial poles.9 As it bends, the cavities of the atrial and ventricular chambers balloon from the linear tube,10 with the atrial appendages in the mammalian heart ballooning in parallel from the atrial component of the primary tube, but with the apical components of the ventricles ballooning in series from the ventricular part of the developing heart (Fig. 17–3A). The molecular signatures of the developing myocardial components are well seen in the mouse heart on the 12th day of development, albeit the apical trabecular component of the right ventricle is only just beginning to balloon at this stage (Fig. 17–3B,C). The evidence in the mouse heart, now known to be replicated during development of the human heart, shows that the areas of primary myocardium are extensive. It is from these areas that, with time, there will be formation of the components of the so-called conduction system as recognized in the definitive heart.11
Figure 17–3.
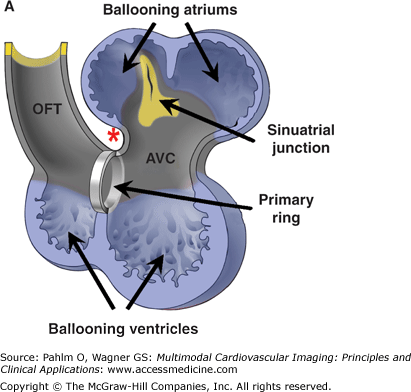
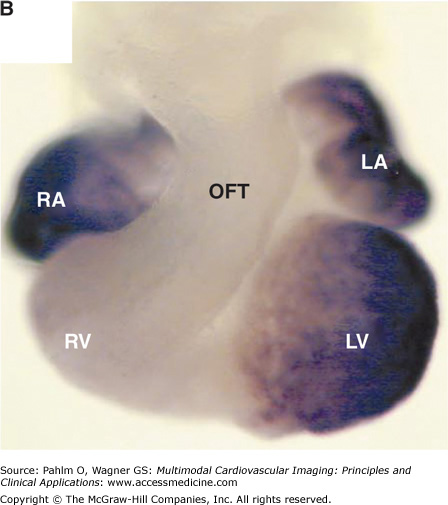
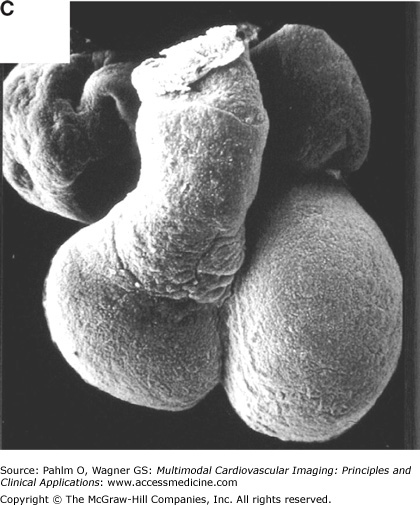
The basic plan of the chamber-forming heart in the mammalian embryo. A. We show schematically the arrangement of the locally differentiating atrial and ventricular chambers (shown in blue) and the primary myocardium (shown in gray). In this scheme, the outflow tract (OFT) is tilted away to show the relationships of the atrial pouches, the primary interventricular ring, and the atrioventricular canal (AVC). Note the continuity of the primary myocardium of the sinuatrial junction, the atrioventricular canal, and outflow tract at the inner curvature (asterisk). B. We show the mouse embryonic heart at embryonic day 11½, stained as a whole mount following in situ hybridization for the atrial natriuretic factor (ANF). This marks the differentiating chamber myocardium of the right atrial (RA) and left atrial (LA) appendages and the right ventricle (RV) and left ventricle (LV). At the stage shown, the expression of ANF in the right ventricle is seen only dorsally. C. We show a scanning electron micrograph of the human embryonic heart at a comparable stage of development. Note the essential similarity in the arrangement of the ballooning chambers and the location of the atrial appendages to either side of the outflow tract.
Morphologic Recognition of the Postnatal Conduction Tissues
As we will discuss, our studies on the developing heart, as yet unpublished, showed the location of the remnants of the initial primary myocardium, now providing insights into the location of arrhythmic substrates in the postnatal heart. The parts of the primary myocardium, including those of the sinus venosus persisting as the components of the so-called cardiac conduction tissues in the definitive postnatal heart, can be recognized morphologically based on the criteria established by Aschoff12 and Mönckeberg13 at the beginning of the twentieth century.12,13 The conduction tissues, according to these criteria, should be histologically distinguishable from surrounding myocardium, should be traceable from one section of the specimen to another, and, if forming tracts, should be insulated from the other myocardium by connective tissue. Thus, all different components of the cardiac conduction tissues in the definitive postnatal heart (Fig. 17–4) can be recognized morphologically using histologic stainings and the fulfillment of the criteria, established by Mönckeberg13 and Aschoff.12 The exemplar of such a conduction tract, as shown by Tawara,14 is the atrioventricular conduction axis (Fig. 17–5A). The sinus and atrioventricular nodes, in contrast, are not insulated from the atrial musculature, thus fulfilling only two of the three criteria (Fig. 17–5B,C). The morphologic features that permit recognition of the regular cardiac nodes also permit additional areas of atrial myocardium to be recognized as being histologically specialized. However, use of these same criteria shows that the pulmonary venous myocardial sleeves and the internodal atrial myocardium, at least in terms of gross histology, are made up of working myocytes.15
Figure 17–4.
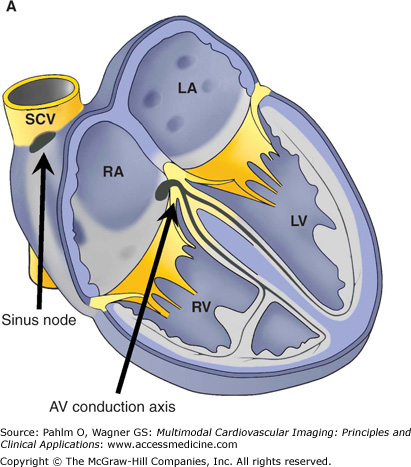
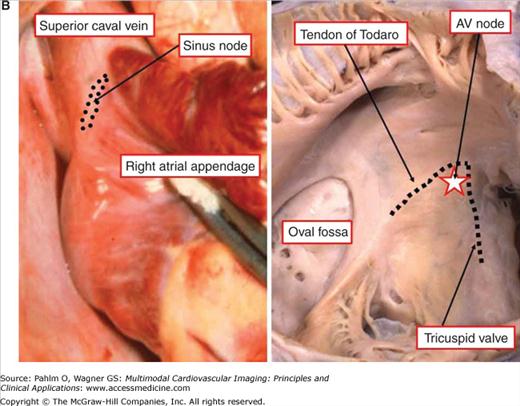
The components of the conduction system in the postnatal human heart. A. The location of the sinus node and the atrioventricular (AV) conduction axis shown in a schematic fashion. B. The gross anatomy of the human heart as related to the cardiac nodes. The sinus node is located at the right side of the junction between the superior caval vein (SCV) and the right atrial appendage. The AV node (star in right side of pane B) is located normally at the apex of the triangle of Koch (dotted line). LA, left atrium; LV, left ventricle; RA, right atrium; RV, right ventricle; SCV, superior caval vein.
Figure 17–5.
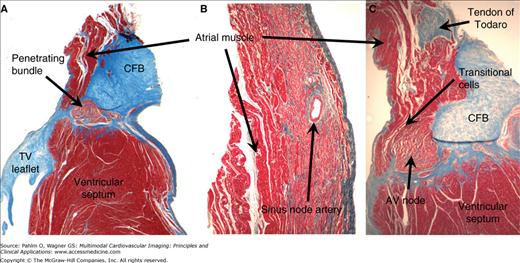
Histologic characteristics of the conduction system in the postnatal human heart. Serial sections through the atrioventricular (AV) conduction axis (A and C) and the sinus node (B) were stained with the Masson’s trichrome, which colors muscle tissues in red and collagen in blue. The penetrating bundle (A), sinus node (B), and AV node (C) are all made up of histologically distinct cells, which can be traced through serial sections. The penetrating bundle is also isolated by the connective tissue from the adjacent working myocardium, which is not the case for the nodes. Note the presence of extensive zones of transitional cells between the working atrial myocardium and the compact part of the AV node in panel C. CFB, central fibrous body. TV, tricuspid valve.
Formation of the Definitive Atrial Chambers
The sinus and atrioventricular nodes and the pulmonary myocardial sleeves are all components of the definitive atrial chambers.16 Knowledge of the development of these components, therefore, requires an appreciation of the morphologic stages involved in formation and separation of the morphologically right and left atrial chambers. It requires, in particular, an understanding of the formation of the atrial septum. Therefore, prior to considering the steps involved in formation of the cardiac nodes themselves, we will review our own understanding of the developmental heritage of the left and right atrial chamber myocardium.
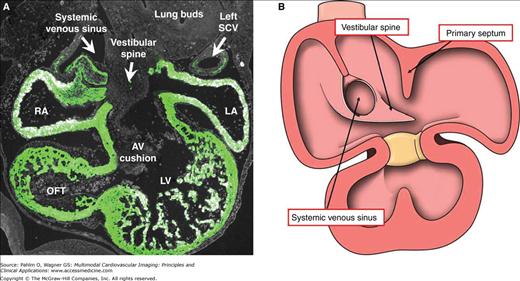
The entire linear heart tube, including the venous pole and the part from which the atrial appendages will balloon, is made up of primary myocardium. The appendages, with walls made of chamber myocardium, balloon in parallel fashion from the primary myocardium. Subsequent to looping of the heart tube, they are positioned rightward and leftward relative to the developing outlet component of the heart (see Fig. 17–3B,C). At the stage at which the systemic venous tributaries enter the atrial component of the tube, which they do in relatively symmetrical fashion, there has been no formation of the lungs. However, it is important to appreciate that the atrial component of the tube remains connected dorsally to the mediastinal mesenchyme through the so-called dorsal mesocardium, where the pulmonary vein will develop (Fig. 17–6). With ongoing development, there is a shift in the relationship of the systemic venous tributaries so that, eventually, they open to the right side of the developing atrial component. In the mouse heart, anatomic boundaries of a discrete intrapericardial systemic venous sinus possessing myocardial walls do not become recognizable until after the tributaries have come to open to the prospective right atrium.4 In the human heart, in contrast, the venous sinus is recognizable morphologically, as a compartment within the pericardial cavity, albeit its walls initially are not myocardial. In both mouse and man, nonetheless, the left-sided venous tributary, which will become the coronary sinus in man, retains its own myocardial walls, passing inferior to the dorsal mesocardial connection as it extends to open in the right atrium (Fig. 17–7). Concomitant with this rightward shift of the atrial connections of the systemic venous tributaries, the dorsal mesocardium itself also undergoes significant remodeling.
Figure 17–6.
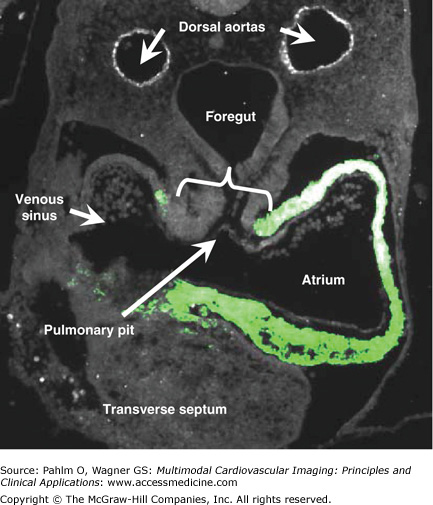
This transverse section is from a young human embryonic heart at Carnegie stage 12 and shows ballooning of the atrial and ventricular chambers. The atrial component of the heart tube is connected to the mediastinal mesenchyme at the ventral surface of the foregut by the so-called dorsal mesocardium (bracket). The section was stained immunofluorescently for myocardial marker SERCA-2a protein, which is revealed by the light green color.
Figure 17–7.
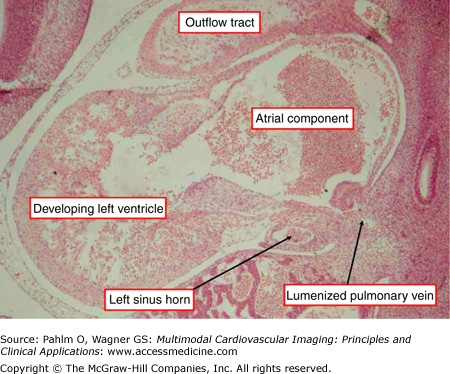
This sagittal section is through a human embryonic heart at Carnegie stage 14, when atrial and ventricular septation is beginning. Note the solitary opening of the newly lumenized pulmonary vein to the atrial component of the heart. The opening is adjacent to the developing left atrioventricular junction, which contains the left sinus horn. Note that the horn, which will become the coronary sinus, possesses its own muscular wall and has no connection with the pulmonary vein. Stained using hematoxylin and eosin.
When first seen, the internal atrial location of the mesocardial connection is marked by the so-called pulmonary ridges, which flank an imperforate pit in the dorsal wall of the undivided atrium.17 With further development, it is possible to trace from this pit an endocardial strand through the mediastinal mesenchyme to the ventral surface of the foregut, where lung buds will eventually develop. With ongoing development, this strand lumenizes to form the pulmonary vein (see Fig. 17–7), which enters the atrium through the dorsal mesocardial connection (Fig. 17–8). At later stages of development, the walls of the newly formed pulmonary vein become myocardial, and neither in mouse nor man do they have any direct connection with the myocardial walls clothing the tributaries of the systemic venous sinus.4,17
Figure 17–8.
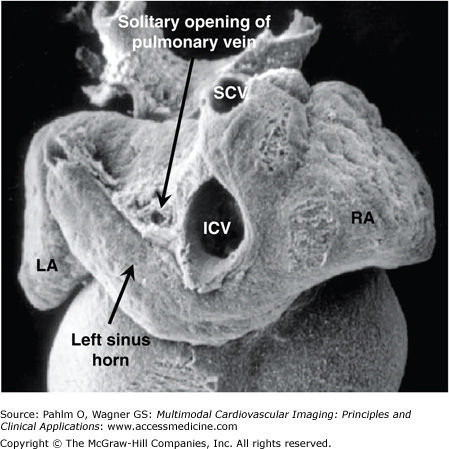
This scanning electron micrograph showing the dorsal aspect of a human heart is from an embryo at the same stage as the section shown in Fig. 17–7. It shows the opening of the pulmonary vein through the persisting dorsal mesocardial connection (dorsal mesenchyme) above the left sinus horn (future coronary sinus) and left of the joining of the superior caval vein (SCV) and inferior caval vein (ICV). LA, left atrium; RA, right atrium.
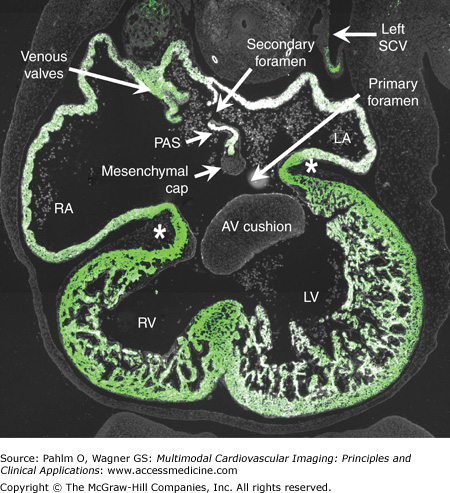
Concomitant with the rightward shift of the tributaries of the systemic venous sinus, septation of the atrial component of the developing heart commences. This is achieved initially by growth toward the atrioventricular canal of a crescentic muscular shelf, the primary atrial septum, or “septum primum.” As it grows toward the atrioventricular canal, which undergoes division by formation of two atrioventricular endocardial cushions, there is a space between the leading edge of the developing septum, which carries a mesenchymal cap, and the atrial surfaces of the cushions. This space is the primary atrial foramen, or “foramen primum” (Fig. 17–9). The foramen is closed by union of the mesenchymal cap on the leading edge of the primary septum and the extracardiac dorsal mesenchyme, or vestibular spine, with the endocardial cushions.18 By the time of fusion, the upper edge of the septum has already broken down to form the secondary atrial foramen, or “foramen secundum” (see Fig. 17–9). As the primary septum fuses with the cushions to close the primary foramen, it completes the confinement of the pulmonary venous return to the left atrium. During this period, the right pulmonary ridge becomes very pronounced and now is recognizable as the vestibular spine, or “spina vestibuli,”19 also known as the dorsal mesenchymal protrusion20 (Fig. 17–10). The area occupied initially by the spine and the mesenchymal cap most probably becomes the extensive muscular anteroinferior rim of the oval foramen. This process of formation of the anteroinferior rim of the oval fossa, as we will see, is particularly important in forming the atrial septal connections of the atrioventricular node, albeit the mechanisms of formation have yet to be established.
Figure 17–9.
This transverse section is from a human embryo at Carnegie stage 16, when atrial septation is being completed. The section was stained immunofluorescently for myocardial marker troponin I, which is shown by the light green color. The primary atrial septum (PAS) is well seen, with the mesenchymal cap on its leading edge, just prior to closure of the primary atrial foramen. The secondary foramen has already appeared at the atrial roof. Note the location of the venous valves, which mark the boundaries of the systemic venous sinus, now exclusively committed to the developing right atrium (RA). There is a muscular continuity between atriums and ventricles via the atrioventricular (AV) canal musculature (asterisks). The AV cushions have already separated the lumen of the AV canal into separate streams. LA, left atrium; LV, left ventricle; RV, right ventricle; SCV, superior caval vein.