Fig. 8.1
Range of acetabular dysplasia seen in hip dysplasia. The right panel shows a mild acetabular dysplasia with no evidence of hip subluxation. The left panel shows a severe acetabular dysplasia with subluxation of the hip and an acetabular rim fracture
Femoral Deformity
Although less commonly treated than acetabular deformities, femoral deformities are commonly present in patients with hip dysplasia. Deformities of femoral version and femoral head/neck morphology are most apparent. Dysplastic hips generally have increased femoral anteversion compared to non-dysplastic hips. Several studies have demonstrated average increases of 10–20° of femoral anteversion compared to controls (which average approximately 15°). However, the degree of femoral anteversion in dysplastic hips is extremely variable and can even include femoral retroversion [8]. An increased valgus orientation (increased neck-shaft angle) to the femoral neck is present on AP pelvis radiographs in almost half of cases [9]. However, in some cases apparent coxa valga may be projectional and not truly present. Noble et al. [10] demonstrated that this valgus orientation of the femoral neck is commonly seen in dysplastic hips with a normal neck-shaft angle due to an increased femoral anteversion when the femoral neck is not placed perpendicular to the plane of the AP pelvis radiograph.
Nearly 75 % of hips with acetabular dysplasia have evidence of aspherical femoral heads or insufficient femoral head–neck offset (Fig. 8.2). Abnormalities of the femoral head–neck junction in dysplastic hips can resemble typical femoral deformities seen in FAI. While impingement can occur at the extremes of motion in dysplastic hips, it is not generally thought to play a major role in the pathophysiology of hip dysplasia. However, impingement after redirectional acetabular osteotomies has been recognized and is influenced by the presence of dysplastic femoral morphologies. Dysplastic hips commonly have smaller femoral heads, femoral necks, and femoral intramedullary canals than non-dysplastic hips. Significant rotational deformities of the proximal femur are commonly present as well, including relatively more posterior location to the greater trochanter.
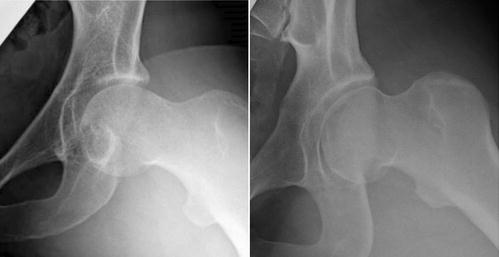

Fig. 8.2
The range of femoral head and head–neck offset deformity seen in acetabular dysplasia. The left panel shows a decrease in head–neck offset seen in a mildly dysplastic hip, while the right panel shows a femoral head deformity in a hip with significant acetabular dysplasia
Natural History
In some series, developmental hip dysplasia remains the most common cause of osteoarthritis in young adults undergoing total hip arthroplasty, accounting for nearly half of all cases [2, 3]. Cooperman et al. [11] demonstrated the development of osteoarthritis in all hips with a LCEA less than 20° at 22-year follow-up. Similarly, Murphy et al. investigated the long-term outcome of the contralateral hip in patients undergoing arthroplasty due to hip dysplasia. Osteoarthritis developed by the age of 65 in all hips with a LCEA less than 16° or an acetabular inclination of greater than 15°. The natural history of hips with borderline acetabular dysplasia (LCEA 20–25°) is more controversial. It appears that some hips with borderline dysplasia develop osteoarthritis, while others do not. Further research is needed to clarify the natural history in this population.
Delayed gadolinium-enhanced magnetic resonance imaging of cartilage (dGEMRIC) has been introduced as a measure of cartilage biochemistry and has been shown to correlate with pain and degree of acetabular deformity in dysplastic populations [12]. dGEMRIC has been shown to be a sensitive means of detecting early degenerative changes not seen on radiographs in this population. A dGEMRIC index of less than 390 ms is generally indicative of early osteoarthritis. Jessel and colleagues [13] demonstrated using dGEMRIC studies that degenerative changes were more strongly correlated with anterior acetabular deficiency, than lateral acetabular deficiency.
Pattern of Articular Damage
Klaue et al. [14] provided an improved understanding of the pathological process of dysplastic hips in their description of “acetabular rim syndrome.” Biomechanical alterations in these hips produce dynamic hip instability and subsequent anterosuperior acetabular rim overload that may lead to acetabular labral and chondral injury and eventual osteoarthritis. Biomechanical analyses have demonstrated increased peak contact stresses at the acetabular rim in dysplastic hips. Elevated contact stresses are due to multiple factors including a lateralized hip center, increased body-weight level arm, and decreased acetabular surface area. The acetabular labrum has a weight-bearing role in the dysplastic hip, unlike the non-dysplastic hip, and generally becomes hypertrophic in response to this stress (Fig. 8.3). This makes the acetabular labrum more prone to labrochondral separation or tearing. Subsequent loss of labral function can lead to increased overload of the acetabular rim and degenerative changes. Ross et al. [15] reported acetabular labral tears and anterosuperior rim articular cartilage lesions in over two-thirds of patients undergoing combined hip arthroscopy and periacetabular osteotomy. Hips with a LCEA less than 15° were more likely to have major articular cartilage damage.
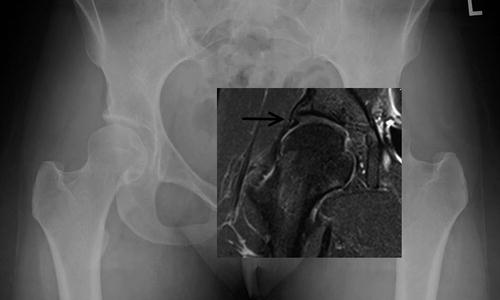

Fig. 8.3
Patient with acetabular dysplasia seen on radiograph. On MRI, the acetabular labrum and cartilage is enlarged
Slipped Capital Femoral Epiphysis
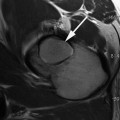
SCFE involves an injury to the immature proximal femoral physis leading to varying degrees of displacement of the femoral neck relative to the epiphysis. The femoral metaphysis generally displaces anteriorly and externally rotates, leading to relative posterior displacement of the epiphysis (Fig. 8.4). Appropriate timely clinical diagnosis and treatment is important to minimize the degree of deformity present. SCFE is commonly classified based on acuity of symptoms [acute (<3 weeks) or chronic (>3 weeks)] and ability to bear weight with crutches (stable or unstable) at presentation. Loder et al. [16] reported no cases of osteonecrosis in stable slips, compared to a 47 % rate of osteonecrosis in unstable slips. However, clinical categorization of stable and unstable slips has been shown to poorly correlate with intraoperative assessment of stability [17, 18].
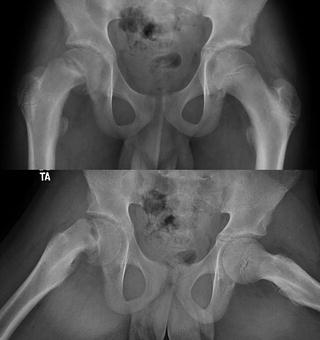

Fig. 8.4
Slipped capital femoral epiphysis seen in the right hip. The femoral neck displaces anteriorly and is externally rotated relative to a femoral head that is in place
Historically, SCFE has been most commonly treated with in situ fixation, as attempts at reduction have been associated with high rates of avascular necrosis. However, advances in surgical technique, including surgical hip dislocation with an extended retinacular flap, allow for protection of the blood supply to femoral head during reduction and have been advocated in some cases [19] (Fig. 8.5). Residual proximal femoral deformity after in situ fixation of a SCFE has been reported to undergo variable degrees of remodeling. Long-term follow-up studies of SCFE have generally shown good hip function, but with significant self-limitation of activities. Recent evidence suggests that FAI occurs in patients with residual deformity and may lead to acetabular chondral and labral damage. Femoral head–neck osteoplasty (Fig. 8.6) has been utilized to address mild residual deformity, while proximal femoral osteotomy (Fig. 8.7) is required for more severe cases. Further research is needed to determine if the long-term outcomes of these procedures alter the natural history of the disease.
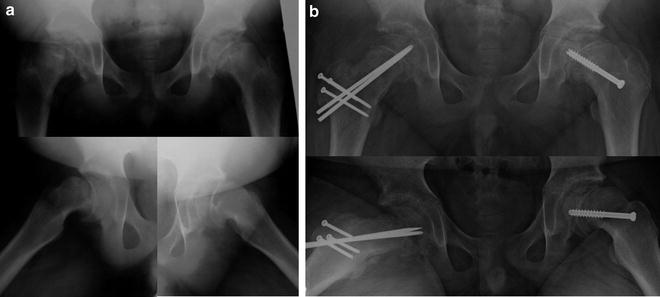
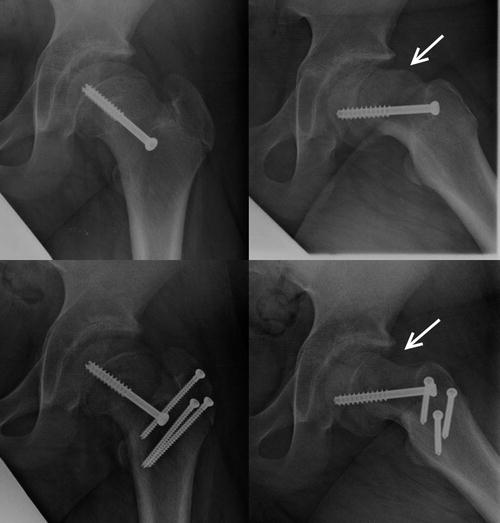
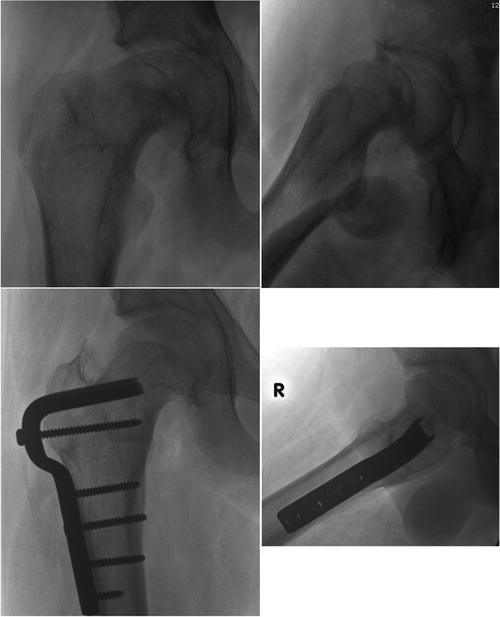

Fig. 8.5
Patient with bilateral slipped capital femoral epiphysis (a). The right hip (b) underwent a modified Dunn procedure to realign the femoral head onto the femoral neck. The milder left hip underwent in situ screw fixation

Fig. 8.6
The anterior metaphyseal prominence seen on the top right panel after in situ fixation was removed (osteoplasty) via a surgical dislocation approach

Fig. 8.7
Severe healed SCFE deformity (top panels) corrected via an intertrochanteric flexion osteotomy and an osteoplasty (bottom panels)
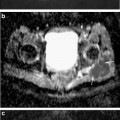
MRI plays a role in the detection of the early occult SCFE with normal plain radiographs (pre-slip). In these cases, MRI demonstrates physeal widening, adjacent bony edema, joint effusion, and synovitis (Fig. 8.8). MRI has a limited role in the presence of radiographic evidence of deformity. MRI may play a role in the detection and characterization of subsequent osteonecrosis, but its role is limited by the degree of metallic artifact present postoperatively.
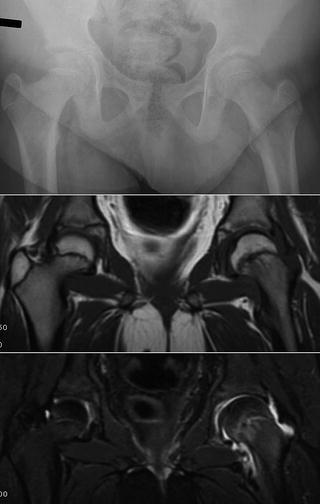

Fig. 8.8
Patient with left hip pain. Plain radiographs show minimal evidence of a SCFE. The T1 weighted image (middle panel) shows some physeal widening in the left hip. The T2 weight image (bottom panel) shows edema around the physis
Epidemiology
SCFE is among the most common pediatric hip disorders with a reported incidence of 10.8 per 100,000 children in the United States in 2000, with a mean age at presentation of 12.1 years [20]. SCFE more commonly occurs in males (13.4/100,000) compared to females (8.1/100,000). The cumulative risk among males approaches 1 per 1,000. The increased prevalence in males is likely due to hormonal factors during puberty. Estrogen generally results in strengthening of the physis, while testosterone decreases the strength of the physis. Mean age at presentation was 12.7 years in males, compared to 11.1 years in females. While the chronologic age of patients with SCFE is variable, the physiologic age has been demonstrated to be narrower [21]. Bilateral SCFE is present at presentation in approximately 20 % of cases, while an additional 10–20 % develop a contralateral SCFE at a later point. Obesity is currently recognized as the most common risk factor for development of SCFE, with more than 80 % of all patients with SCFE being obese. Trends of increasing obesity in children have been mirrored by increasing rates of SCFE. SCFE is more common among African-American and Hispanic populations, reflecting increased rates of obesity in these populations. Other recognized risk factors include endocrine disorders, renal osteodystrophy, and previous radiation therapy. Endocrine abnormalities are present in 5–8 % of SCFEs, including hypothyroidism, panhypopituitarism, growth hormone deficiency, and hypogonadism.
Femoral Deformity
Subtle changes in femoral morphology may predispose some individuals to the development of SCFE. Decreased femoral anteversion is commonly present in hips with SCFE at presentation and may play a role in the development of SCFE due to increasing shear force across the physis. Pritchett et al. [22] reported that relative femoral retroversion in SCFE patients results in increased shear stress on the physis by 3.3 times body weight in the sagittal plane during fast walking. Gelberman et al. [23] reported a mean femoral neck anteversion of 1° as measured by CT in a cohort of 39 slips, compared to standard reference values of 15–20° in this age group. Similar levels of anteversion were present in slips at presentation and at follow-up after operative treatment. Additionally, decreased femoral anteversion has been noted in populations of obese patients with an average of 0° of anteversion compared to 11° in normal contrals. This association may partially explain the link of obesity and SCFE. Similarly, a mild varus alignment of the femoral neck results in a more vertical orientation of the physis and may increase shear force and contribute to the development of SCFE. The physeal orientation in the contralateral hips of patients with SCFE has been shown to be 8–11° more oblique than normal.
Anterior displacement of the proximal femoral metaphysis is the primary deformity present in SCFE. This has been traditionally described as posterior displacement of the proximal femoral epiphysis, despite the unchanged relationship between the epiphysis and the acetabulum. Medial displacement of the epiphysis is variably present on anteroposterior radiographs, but may be a result of the relatively externally rotated posture of the leg that is commonly present. The severity of the deformity is commonly classified using measurement of the Southwick slip angle on frog-leg lateral radiographs (Fig. 8.9), in which the femoral epiphyseal-shaft angle is measured on the affected side and subtracted from the measurement of the contralateral normal hip. SCFE is classified as mild with angle 0–30°, moderate 30–60°, and severe 60°.
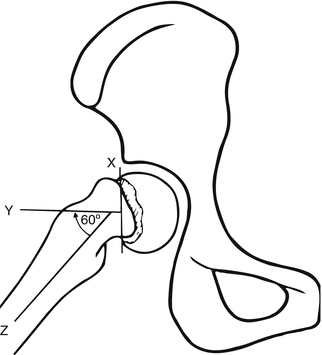

Fig. 8.9
The Southwick slip angle measured on the frog lateral view
The metaphyseal prominence in the residual SCFE is considered the major residual deformity and may result in FAI. Rab characterized the residual SCFE deformity and its role in FAI utilizing three-dimensional modeling of walking and sitting positions [24]. Two types of FAI were noted: “impaction” and “inclusion.” Impaction-type impingement occurs in moderate to severe deformities where a large metaphyseal prominence impacts the acetabular rim preventing further motion. This type of impingement results in severe alterations of range of motion, including the classic obligatory external rotation that occurs with hip flexion. Additionally, external rotation of the leg may be required during walking to prevent impingement. Inclusion-type impingement occurs with milder deformities where a smaller metaphyseal prominence is able to enter the acetabulum but may result in chondrolabral damage, similar to the mechanism seen in cam-type FAI. In a CT-based model of bony impingement, Mamisch et al. [25] demonstrated that the relative head–neck offset, in addition to degree of slip, influences range of motion to impingement.
In 1926, Key [26] first described radiographic evidence of remodeling after SCFE, including resorption of the anterior-superior metaphyseal bone and new bone formation along the posterior-inferior neck. Remodeling of the metaphyseal prominence in SCFE after in situ fixation has been demonstrated by multiple studies, but is variable. Remodeling potential is generally greater in individuals with an open proximal femoral physis and significant remaining growth potential. O’Brien et al. [27] reported on the remodeling of 12 patients with moderate or severe slips treated with in situ fixation. Remodeling was observed in 83 % of hips, generally in the presence of an open triradiate cartilage. However, radiographic evidence of remodeling in these patients would be viewed as incomplete by modern standards. Jones et al. [28] described a classification system for residual deformity of the anterior head–neck junction. Type A hips demonstrate a normal transition from the convexity of the femoral head to the concavity of the anterior femoral neck. In type B hips the transition from femoral head to femoral neck is straight. Type C hips demonstrate convexity of the head–neck junction with the anterior margin of the femoral head projecting posterior to the anterior prominence of the neck (Fig. 8.10). Unlike by modern standards, type A and B hips were defined as completely remodeled (90 % of mild, 50 % of moderate or severe slips). Siegel et al. [29] demonstrated remodeling reductions in slip angle from 44° to 30° as measured by computed tomography at 2 years postoperatively. Significant gains in flexion and internal rotation range of motion generally occurred by 6 months postoperatively without associated changes in femoral version. Changes in range of motion after 6 months postoperatively were minimal. While multiple studies demonstrate the remodeling does occur after in situ fixation of SCFE, the remodeling is generally incomplete with significant residual deformity present [30].
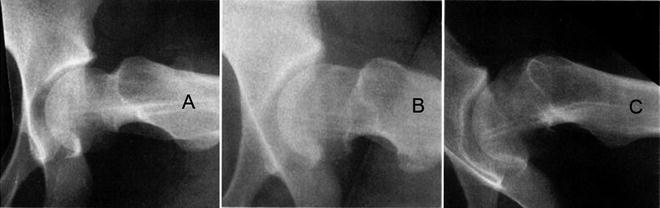

Fig. 8.10
Metaphyseal remodeling after SCFE can be classified as Jones type: (A) normal convexity in head–neck junction, (B) straight transition from head to neck, and (C) convex projection of the head–neck junction
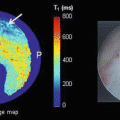
Femoral deformities in cam-type FAI have been speculated to be the result of mild or subclinical slips [31]. However, recent evidence suggests that while the deformity in cam-type FAI may result from physeal stresses, the deformity does not appear to occur from displacement of the epiphysis as seen in SCFE. Utilizing MRI, Siebenrock et al. [32] demonstrated that the typical anterosuperior cam deformity is a result of abnormal extension of the proximal femoral physis, compared to controls (Fig. 8.11). Additionally, this study established that the proximal femoral epiphysis is otherwise normally oriented relative to the femoral neck, without evidence of posterior epiphyseal displacement seen in SCFE.
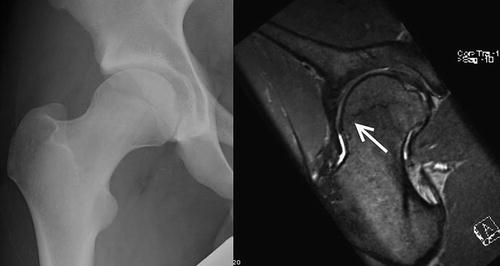

Fig. 8.11
Physeal extension seen on the MRI scan of a patient with cam impingement. Deformity is distinctly different from a SCFE
Acetabular Deformity
The presence of acetabular deformity in patients with SCFE has been variably reported. Unlike other pediatric hip disorders, SCFE generally occurs at an age when limited remodeling capabilities of the acetabulum exist. Acetabular overcoverage or retroversion could play a role in the etiology of SCFE in some patients, as well as worsening FAI occurring in patients with residual deformity. Kitadai et al. reported a slightly increased acetabular coverage in patients with SCFE compared to matched controls (LCEA 37° vs. 34°). Several studies have showed no significant difference in acetabular version between involved and uninvolved hips in patients with SCFE. Monazzam et al. [33] demonstrated increased prevalence of cranial acetabular retroversion on CT, increased rates of acetabular overcoverage in hips with SCFE, as well as contralateral unaffected hips. Utilizing plain radiographs, Sankar et al. [34] found significantly increased coverage (LCEA 33° vs. 20°) and prevalence of acetabular retroversion (positive crossover sign 78 % vs. 21 %) in the contralateral hip of patients with SCFE compared to a control group.
Natural History
Rates of osteoarthritis in mild slips treated with in situ fixation at 31- to 41-year follow-up have been reported from 16 to 64 %, with 7–10 % rates of severe osteoarthritis. Similarly, in severe slips rates of osteoarthritis were reported from 60 to 100 %, with 33–40 % rates of severe osteoarthritis. Carney et al. [35] reported a series of 155 hips with SCFE at a mean follow-up of 41 years. The authors concluded the natural history in this population was characterized by “mild deterioration related to the severity of slip and complications.” Osteonecrosis and chondrolysis occurred more commonly after reduction attempts. Twenty-eight percent of patients underwent further surgery (generally arthroplasty) at a median of 28 years postoperatively. Further surgery was more common with more severe slips (12 % mild, 30 % moderate, 53 % severe). Fraitzl et al. [36] reported on a cohort of 16 patients with average age of 28 years after in situ pinning of mild slips. Tegner activity scores averaged 5.2, with more than half be 4 or less (implying minimal sporting activities). Residual deformity was more prominent at the lateral head–neck junction (mean alpha 86°), than the anterior head–neck junction (mean alpha 55°). Goodman et al. [31




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree