Devices and Postoperative Appearance
QUESTIONS
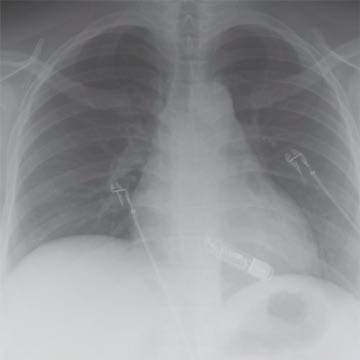
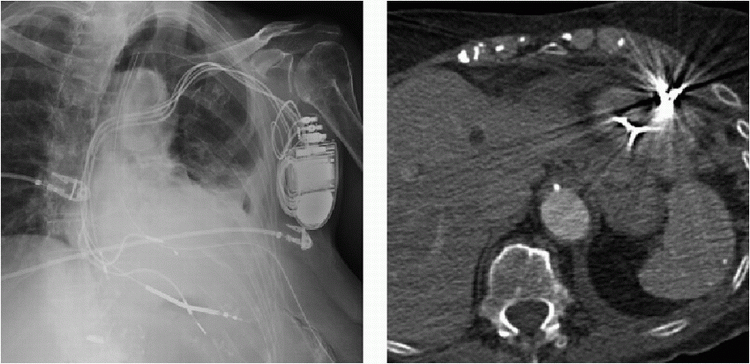
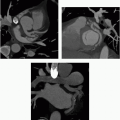
1a A 35-year-old male has a history of syncope. There is no family history of sudden death. The patient is scheduled for a cardiac MRI, and the technologist asks you to review a screening chest radiograph.
|
What is the finding on the radiograph?
A. Repeat CXR without device in patient’s pocket
B. Previous pacemaker with leads removed
C. Loop recorder
D. External ICD
View Answer
1a Answer C. The chest radiograph demonstrates a radiopaque device overlying the heart, compatible with an implantable loop recorder, and is typically contained in the anterior soft tissues of the chest.
Implantable loop recorders (ILR) are useful in detecting undiagnosed recurrent arrhythmic episodes particularly in unexplained syncope with a significantly higher diagnostic rate than other conventional tests.
ILR not only allows for prolonged monitoring without external electrodes (up to 3 years) but they also have the ability to autoactivate when an arrhythmia is present, allowing episodes to be captured independent of manual patient activation of the device. Once an episode is recorded, the memory is archived by the patient or a relative by applying a nonmagnetic handheld activator. Given prolonged electrocardiographic monitoring, loop recorders can provide more accurate correlations between a patient’s symptoms and documented abnormalities in heart rhythm.
Reference: Subbiah RN, Gula LJ, Klein GJ, et al. Ambulatory monitoring (Holter, event recorders, external, and implantable loop recorders and wireless technology). In: Electrical diseases of the heart. 2008: 344-352.
1b After reviewing the previous screening chest radiograph, how would you proceed with the scheduled cardiac MRI?
A. The x-ray does not have any abnormalities to be concerned with.
B. Discuss the issue with the patient and proceed with the cardiac MRI.
C. Discuss the issue with the patient and postpone until you can discuss with the primary provider.
D. Discuss the issue with the patient and cancel the study because it is contraindicated.
View Answer
1b Answer B. Studies investigating the effect of scanning implantable loop recorders (ILR) in an MRI environment demonstrate no significant translational movement or dislodgement of ILRs in relation to exposure to long-bore and short-bore 1.5 T MRI systems. Thus, MRI scanning of ILR patients can be performed without harm to the patient or device. However, artifacts that could be mistaken for a tachyarrhythmia are seen frequently and should not be interpreted as pathology.
References: Shellock FG, Tkach JA, Ruggieri PM, et al. Cardiac pacemakers, ICDs, and loop recorder: evaluation of translational attraction using conventional (“long-bore”) and “short-bore” 1.5 and 3.0 tesla MRI systems: safety. J Cardiovasc Magn Reson 2003;5(2):387-397.
Wong JA, Yee R, Gula LJ, et al. Feasibility of magnetic resonance imaging in patients with an implantable loop recorder. Pacing Clin Electrophysiol 2008;31(3):333-337.
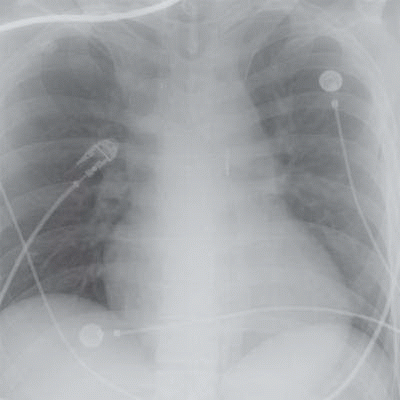
2a A chest radiograph was performed for a patient in the cardiac intensive care unit to check intra-aortic balloon pump placement (below).
|
Which of the following are appropriate indications for IABP placement?
A. Aortic dissection
B. Acute tricuspid incompetence
C. Aortic insufficiency
D. Acute mitral incompetence
View Answer
2a Answer D. The chest radiograph demonstrates an intra-aortic balloon pump with its tip terminating in the descending thoracic aorta, adequately placed.
Intra-aortic balloon pumps (IABP), initially introduced in the 1960s, remain the most widely used form of mechanical circulatory support for patients with critical cardiac disease. As the IABP balloon expands, the volume displacement of blood, which occurs both proximally and distally in the ascending and proximal descending aorta, is termed “counterpulsation.” Effectively, balloon inflation in diastole and then rapid deflation in systole results in a decrease in systolic blood pressure and an increase in diastolic pressure. The result is afterload reduction in systole and augmentation of aortic root and coronary artery pressure in diastole, when coronary perfusion pressure is maximal.
The available clinical evidence supports intra-aortic balloon pump placement in cases of cardiogenic shock or refractory angina. Other indications such as mechanical complications of myocardial infarction (i.e., acute mitral regurgitation and ventricular septal defect), intractable arrhythmia, and refractory heart failure are less common, yet generally accepted indications for IABP support.
IABP placement is contraindicated in patients with aortic insufficiency because it worsens the magnitude of regurgitation. IABP insertion should not be attempted in case of suspected or known aortic dissection because inadvertent balloon placement in the false lumen may result in extension of the dissection or even aortic rupture. Similarly, aortic rupture can occur if IABP is inserted in patients with sizable abdominal aortic aneurysms. Patients with end-stage cardiac disease should not be considered for IABP unless as a bridge to ventricular assist device or cardiac transplantation.
References: Tsagalou EP, Drakos SG, Tsolakis E, et al. Intraaortic balloon pump in the management of acute heart failure syndromes. In: Mebazaa A, Gheorghiade M, Zannad FM (eds.). Acute heart failure. London: Springer London, 2008:671-683.
White JM, Ruygrok PN. Intra-aortic balloon counterpulsation in contemporary practice—where are we? Heart Lung Circulation 2015;24(4):335-341.
2b On a follow-up chest radiograph, the tip of the IABP lies just beyond the left subclavian artery. What would you recommend?
A. IABP is adequately positioned and may remain in place.
B. The IABP lies too distal and should be advanced.
C. The IABP lies too proximal and should be retracted.
D. The IABP should be removed.
View Answer
2b Answer C. IABP placement is important for successful diastolic augmentation of coronary perfusion. The IABP catheter is inserted percutaneously into the femoral artery through an introducer sheath with a modified Seldinger technique. Once vascular access is obtained, the balloon catheter is inserted and advanced, usually under fluoroscopic guidance, into the proximal descending thoracic aorta, with its radiopaque tip 2 to 3 cm distal to the origin of the left subclavian artery (at the level of the carina).
If the tip is too proximally placed, the balloon may obstruct the great vessels of the aortic arch. If the tip is too distally placed, then the IABP may not be effective enough in increasing coronary blood flow and may also obstruct the splanchnic vessels.
References: Krishna M, Zacharowski K. Principles of intra-aortic balloon pump counterpulsation. Continuing Educ Anaesthesia Crit Care Pain 2009;9(1):24-28.
White JM, Ruygrok PN. Intra-aortic balloon counterpulsation in contemporary practice—where are we? Heart Lung Circulation 2015;24(4):335-341.
3 Which of the following devices would present a contraindication to perform cardiac MR?
A. Prosthetic heart valve
B. IVC filter
C. Coronary stent
D. Implantable cardiac defibrillator
View Answer
3 Answer D. Patients have to be carefully screened to exclude ferromagnetic implants or ferromagnetic foreign bodies due to effects of the magnetic field. Implantable devices are generally categorized as “MRI safe,” meaning they are safe under any, even future, MRI conditions; “MRI conditional,” meaning that the implant is safe under certain conditions, which have to be specified in detail for any given device; and “MRI unsafe” which includes any item that is known to pose hazards in all MRI environments.
Passive implants may interact mainly with the main magnetic field (force and torque effects) and RF field, which may induce heating. Most of currently implanted stents, heart valves, sternal wires, cardiac closure, occluder devices, filters, embolization coils, and screws are MRI conditional. Most tested implanted coils, filters, stents, and grafts are unlikely to move or become dislodged as a result of exposure to MRI systems operating at 3 tesla (T) or less. It is unnecessary to wait an extended period of time after surgery to perform an MRI procedure in a patient with a “passive” metallic implant that is made from a nonferromagnetic material. However, new coils, stents, filters, and vascular grafts are developed on an ongoing basis, and attention to the manufacturer’s product information or reference manuals is always recommended.
The majority of coronary stents, aortic endografts, and IVC filters are made from nonferromagnetic or weakly ferromagnetic materials. It is generally believed that additional anchoring of these implants into vessel walls occur over 6 to 8 weeks, primarily due to tissue ingrowth. All passive implants, which are nonferromagnetic, may undergo MRI procedures at 3 T or less at any time after implantation. Any implant, which is weakly ferromagnetic, should be scanned preferably 6 weeks after implantation and should be weighed on a case-by-case basis.
The Zenith AAA endovascular graft stent demonstrates high magnetic forces and has been labeled as “MRI unsafe.”
Active implants may react on the different electromagnetic fields of an MRI unit, and safety evaluations are much more complicated. Implantable cardioverter-defibrillators (ICDs) are among these devices. ICDs contain metal with variable ferromagnetic qualities, as well as complex electrical systems, and additionally consist of one or several leads implanted into the myocardium. There is potential for movement of the device, programming changes, asynchronous pacing, activation of tachyarrhythmia therapies, inhibition of pacing output, and induced lead currents that could lead to heating and cardiac stimulation.
Current recommendations consider the presence of a pacemaker or ICD a strong relative contraindication to routine MRI examination. Alternative diagnostic tests should be primarily considered, and MR imaging should only be considered when there is a strong clinical indication and in which the potential benefit to the patient clearly outweighs the risks to the patient.
Reference: Levine GN, Gomes AS, Arai AE, et al. Safety of magnetic resonance imaging in patients with cardiovascular devices: an American Heart Association Scientific Statement From the Committee on Diagnostic and Interventional Cardiac Catheterization, Council on Clinical Cardiology, and the Council on Cardiovascular Radiology and Intervention: endorsed by the American College of Cardiology Foundation, the North American Society for Cardiac Imaging, and the Society for Cardiovascular Magnetic Resonance. Circulation 2007;116(24):2878-2891.
A. Pneumopericardium
B. Artificial heart
C. Mitral and tricuspid valve repair
D. Ventricular assist device
View Answer
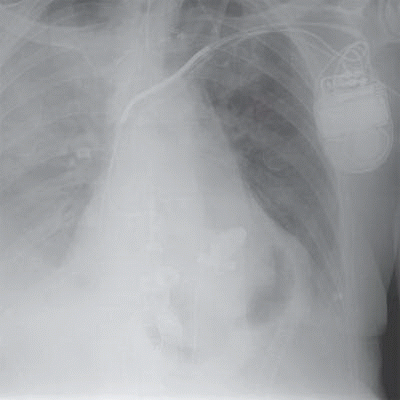
4 Answer B. The radiograph demonstrates sternotomy from cardiac surgery and well-circumscribed areas of lucency in the region of the ventricles, which correlate with the ventricular bellows of a total artificial heart. Prosthetic valves are also observed, which are anastomosed to the native atria.
The total artificial heart (TAH) is a biventricular mechanical assist device that is implanted following definitive explantation of the patients failing ventricles. The duration of implantation varies depending on a particular patient’s medical condition and the eventual availability of a human heart for orthotopic transplantation. Contraindications for implantation included chronic cardiac cachexia, advanced physiologic age, chronic failure of end organs incompatible with recovery, anticipated to be impossible to recover to transplant candidate status, and judged to have inadequate mediastinal size for the TAH.
Chest radiography is routinely used immediately after TAH implantation, during hospitalization, and until the time of orthotopic transplant to assess the device and monitor for complications. Similar to the case presented here, four mechanical valves are positioned in nonanatomic locations. Air-filled right and left ventricular spaces may mimic the appearance of intracavitary air, pneumomediastinum, pneumopericardium, and abscess.
The TAH is associated with a low but significant risk of thrombosis. As soon as hemostasis allows it, patients need to be placed on a stringent anticoagulation regimen. Postoperative hemothorax or hemomediastinum also occurs in a high percentage of TAH recipients. The need for anticoagulation also increases risk for extrathoracic hemorrhage, including subarachnoid or spontaneous retroperitoneal hemorrhage.
References: Copeland JG, Copeland H, Gustafson M, et al. Experience with more than 100 total artificial heart implants. J Thorac Cardiovas Surg 2012;143(3):727-734.
Parker MS, Fahrner LJ, Deuell BPF, et al. Total artificial heart implantation: clinical indications, expected postoperative imaging findings, and recognition of complications. AJR Am J Roentgenol 2014;202(3):W191-W201.
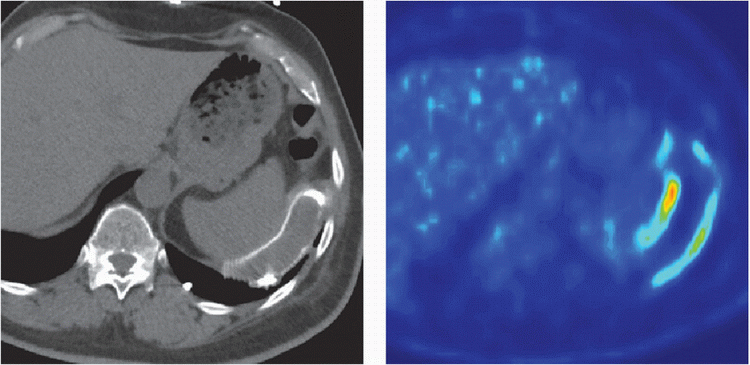
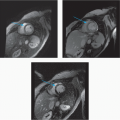
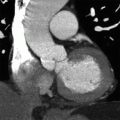
5 Patient is status post ventricular assist device implantation 8 months ago and now presents with the following CT and PET imaging:
|
Which of the following is the most likely diagnosis?
A. LVAD infection
B. Cannula fracture
C. LVAD thrombosis
D. Normal activity
View Answer
5 Answer A. Noncontrast CT demonstrates mild fluid surrounding the outflow cannula that subsequently shows increased metabolic activity on PET imaging. The findings are very suggestive of infection.
The development of left ventricular assist devices (LVADs), first as a bridge to transplant and then as destination therapy, has significantly improved survival and quality of life of patients with end-stage heart failure.
LVAD infections remain among the most frequently encountered adverse events and often lead to significant morbidity and mortality. LVAD-specific infections may be of the hardware itself or the body surfaces that contain them and include infections of the pump, cannula, anastomoses, pocket infections, and the percutaneous driveline or tunnel. Percutaneous driveline infections are the most commonly occurring infections in LVAD patients and may reflect the presence of a deeper infection of the pocket space or pump and/or cannula.
LVAD-related infections include infectious endocarditis, mediastinitis, and sternal wound infection. Evaluation with CT may reveal large valve vegetations and cannula insertion infections. CT may also play a role in characterizing sternal wound infections, particularly to define the extent of deep-seated infection or collection.
References: Hannan MM, Husain S, Mattner F, et al. Working formulation for the standardization of definitions of infections in patients using ventricular assist devices. J Heart Lung Transpl 2011;30(4):375-384.
Lima B, Mack M, Gonzalez-Stawinski GV. Ventricular assist devices: the future is now. Trends Cardiovasc Med 2015;25(4):360-369.
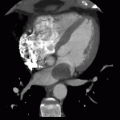
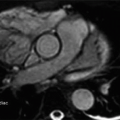
6 Patient with ventricular assist device and worsening heart failure presents with the following CT:
|
Which of the following is the best diagnosis?
A. LVAD infection
B. Cannula fracture
C. LVAD thrombosis
D. Myocardial infarction
View Answer
6 Answer C. Axial image from a cardiac CT demonstrates the inflow (left) and outflow (right) cannulas of a ventricular assist device inferior to the base of the heart. Thrombus is observed obstructing the outflow cannula.
The development of left ventricular assist devices (LVADs), first as a bridge to transplant and then as destination therapy, has significantly improved survival and quality of life of patients with end-stage heart failure.
LVAD thrombosis occurs in 2% to 13% of adult patients with a continuous-flow LVAD. This thrombus may form as an acute event or insidiously over a prolonged period of time. Thrombus in the left ventricle, inflow cannula, pump housing, outflow cannula, outflow graft, or aortic root may produce devastating events that include thromboembolic stroke, peripheral thromboembolism, LVAD malfunction with reduced systemic flows, LVAD failure with life-threatening hemodynamic impairment, cardiogenic shock, and death.
For this reason, patients are placed on anticoagulation therapy with warfarin and antiplatelet therapy with aspirin.
Two types of thrombi may develop in an LVAD. Red thrombus forms as stagnating blood coagulates under low pressure. In contrast, white thrombus constructed primarily from activated platelets forms in areas of turbulence. The distinction between the types of thrombi is critical as red thrombi are best treated with anticoagulation, whereas white thrombi are better managed with antiplatelet agents.
An echocardiographic “ramp study” is performed for diagnostic confirmation, whereby stepwise increases to maximal pump speeds fail to elicit complementary augmentation in pump flow. While variable success with thrombolytic therapy has been reported, definitive treatment usually entails operative pump exchange.
The sensitivity of echocardiography surpasses CT to diagnose LVAD thrombus, especially if three-dimensional echocardiography is available. Yet, echocardiography may fail to diagnose LVAD thrombosis, and cases have been described in which CT, but not echocardiography, identified LVAD thrombus. In addition, CT is not limited by acoustic windows and shadowing. Consequently, when suspicion for LVAD thrombosis is high, a negative imaging study should prompt further investigation with additional techniques.
The definitive therapy for LVAD thrombosis is explanation of the device and cardiac transplantation. Unfortunately, the immediate availability of a compatible donor heart leaves this option as a last resort.
References: Bartoli CR, Ailawadi G, Kern JA. Diagnosis, nonsurgical management, and prevention of LVAD thrombosis: LVAD thrombosis. J Cardiac Surg 2014;29(1):83-94.
Lima B, Mack M, Gonzalez-Stawinski GV. Ventricular assist devices: the future is now. Trends Cardiovasc Med 2015;25(4):360-369.
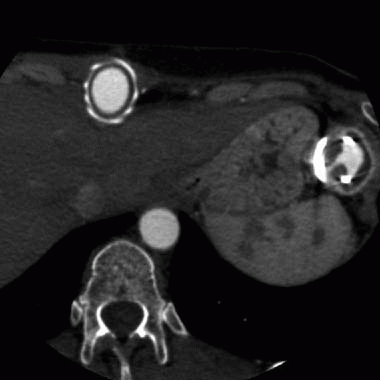
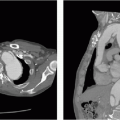
7 A 43-year-old male has a history of ventricular tachycardia and is status post ICD placement 4 months ago now complains of chest pain.
|
Which of the following is the most likely diagnosis?
A. Myocardial infarction
B. Lead perforation
C. Lead fracture
D. Pneumothorax
View Answer




7 Answer B. Portable AP radiograph demonstrates multiple implanted cardiac leads, which tips overly the right ventricle. Further evaluation by CT demonstrates that the tip has migrated beyond the myocardium and terminates outside the heart.
Cardiac perforation after pacemaker or implantable cardioverter-defibrillator (ICD) implantation is an infrequent complication, more frequently seen in the right ventricle but also in the right atrium. Cardiac perforations may present as acute (events occurring within 24 hours after implantation), subacute (occurring 5 days to 1 month after implantation), or delayed manifestations (occurring more than 1 month after implantation).
The most common symptom is pacing or sensing failure. If a lead perforates the myocardium, capture threshold will be increased and sensing threshold will be reduced in general. In some asymptomatic patients with delayed perforation, pacemaker function and electrophysiologic parameters appear normal and thus cannot exclude cardiac perforation. Hemodynamic stability is mainly determined by the development of hemopericardium.
Sharp chest pain during the insertion, evidence of cardiac tamponade with breathlessness, raised jugular venous pressure, falling systemic blood pressure, and cyanosis, is suggestive of hemopericardium that requires emergency pericardiocentesis and possibly cardiac surgical repair. Echocardiography or computed tomography should confirm hemopericardium and may even show the electrode tip in the pericardial space. Signs and symptoms of pericarditis, including a pericardial friction rub, are also suggestive.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree