(1)
Department of Clinical Radiology, Amiri Hospital – Kuwait City, Kuwait City, Kuwait
10.1.1 Diabetic Angiopathy
10.1.3 Diabetic Myonecrosis
10.1.6 The Role of MRI in DM
10.3 Diabetic Syndromes
10.3.1 Alström Syndrome
10.3.2 Bardet–Biedl Syndrome
10.3.4 Prader–Willi Syndrome
10.3.5 Wolcott–Rallison Syndrome
10.3.6 Wolfram Syndrome (DIDMOAD)
10.3.7 Rabson–Mendenhall Syndrome
10.4 Diabetes Insipidus
10.5.1 Hormonal Obesity
10.5.2 Syndromic/Pathologic Obesity
10.5.3 Drug-Induced Obesity
10.5.4 Gastric Banding
10.5.5 Liposuction
10.6.3 Mandibuloacral Dysplasia
10.7 Diabetic Nephropathy
10.7.1 Diabetic Nephropathy
10.7.2 Renal Papillary Necrosis
10.7.5 Emphysematous Cystitis
10.7.6 Emphysematous Pyelonephritis
10.8 Lipomatosis
10.8.1 Intestinal Lipomatosis
10.8.2 Pelvic Lipomatosis
10.8.3 Epidural Lipomatosis
10.9 Hypoglycemia
10.1 Diabetic Hand and Diabetic Foot
Diabetes mellitus (DM) is a chronic metabolic disease that arises due to insulin deficiency (type 1 DM) or insulin receptor insensitivity (type 2 DM). Type 2 DM is more common than type 1.
Diabetic complications arise due to cellular ischemia, angiopathy, peripheral neuropathy, osteopathy, infections, skin changes, and atherosclerosis. The hands and feet are uncommonly affected in diabetes, but when they are affected, it may be severe enough to cost the patient loss of a limb.
Radiology offers great tools for early detection of diabetic complications by ultrasound and MRI. For diabetic foot screening, a Doppler scan is performed to detect arterial flow anomalies. If the Doppler scan shows abnormalities in the vessels, MRI can be done to detect hidden signs of diabetic foot complications. Adults with long-term DM should be annually examined for lower limb vascular abnormalities.
Diabetic Angiopathy
Diabetic angiopathy is divided into two types: microangiopathy and macroangiopathy. Microangiopathy arises due to chronic hyperglycemia that impairs the walls of the microvessels, causing leakage of exudates and blood. Later, these exudates may lead to obstruction of the microvessels causing ischemia. This type is typically seen in diabetic retinopathy and diabetic nephropathy. Macroangiopathy, on the other hand, damages the arterial vessels due to atherosclerosis affecting the coronary, cerebral, and lower limb vessels. Arteriosclerosis occurs 10 years earlier in diabetics than in normal people.
Chronic limb ischemia and compromised vascular supply can lead to tissue necrosis and dry gangrene. This is often complicated by bacterial infection that may cause wet gangrene; this scenario is often seen in the feet. Amputation is the tragic end of severe limb osteomyelitis, extensive lower limb calcifications, and uncontrolled diabetes that suppresses the immune system. Within 2 years of amputation of one leg, the other leg has a 50 % chance of complications that might lead to a 50 % chance of contralateral amputation.
Gangrene can be divided into dry, wet, and infected. Dry gangrene arises due to an occluded artery with a patent vein; tissue liquefaction occurs at a very slow rate. It is seen in senile gangrene (due to atherosclerosis and vascular stasis) and Buerger’s disease (thromboangiitis obliterans). Senile gangrene is seen in 50 % of elderly patients wearing tight shoes and commonly affects the big toe. Wet gangrene arises due to an occluded artery and vein, with rapid tissue liquefaction and sever toxemia. This type is classically seen in DM, crush injuries (accidents), and bedsores. Infected gangrene arises due to bacterial infection and is typically seen in lung abscess, necrotizing fasciitis, synergistic gangrene, and gas gangrene (due to muscular lesion with anaerobic fermentation of the tissues with Clostridium difficile).
As previously mentioned, patients with gangrene are treated by amputation of the gangrenous part of the lower extremity, which can be above or below the knee, depending on the extension of the compromised vascular supply. The amputee may develop stump pain after surgery, which can be attributed to stump infection, inflammation, impaired vasculature, or development of neuromas. A neuroma is a focal, nodular, noncapsulated soft-tissue mass that forms at the distal segment of peripheral nerves after surgery or traumatic avulsion injury. Schwann cells regenerate the peripheral nervous system axons and myelin sheath after trauma. In an amputated limb, regeneration of the nerve axon is unstoppable, because there is no distal end pathway for the regenerated nerve axon to fuse with, resulting in aggregation of the Schwann cells at the stump end, forming a mass of nerve tissue. Postamputation neuromas are usually multiple and may appear 1 month after amputation. Patients typically present with stump pain, usually in the absence of inflammation or stump infection.
Signs on Radiographs
Calcification of pedal vessels occurs in 24 % of diabetic patients, and it is seen radiologically as classic “tramline” or “pipestem” calcification (Fig. 10.1.1).


Fig. 10.1.1
A lateral plain radiograph of a patient with severe diabetic foot shows calcaneal ulcer (hollow arrowhead), osteomyelitis causing bone resorption and necrosis (solid arrowhead), and calcified arteries due to macroangiopathy (arrows)
Signs on MRI
Postamputation neuromas are detected as ovoid, bulbous, or rounded soft-tissue mass expansions at the end of a proximally transected nerve (e.g., peroneal nerve in above-knee amputation) that classically measure 1–2 cm in diameter. The mass shows low T1 and moderately high T2 signal intensity, with a characteristic dark rim seen on both T1W and T2W images due to focal fibrous tissue condensation around the neuroma.
Diabetic Peripheral Neuropathy, Osteopathy, and Infections
Diabetic peripheral neuropathy often affects both hands and feet in a bilateral symmetrical fashion (glove and stocking phenomenon). Loss of the deep knee tendon reflex is the earliest sign of diabetic neuropathy, even before any sensory or motor disturbances manifest. Diabetic neuropathy is attributed to metabolic abnormalities affecting Schwann cells, the myelin-forming cells of the peripheral nervous system.
In the neuropathic diabetic foot, sympathetic denervation is the main pathological injury. Somatic and autonomic denervation causes numbness and loss of heat and pain sensation, along with reduction in the sensation of touch and vibration. Sympathetic denervation causes arteriovenous shunts within hands and feet, causing abnormal increase in the venous flow within the limbs. Moreover, the intracutaneous pressure causes the development of calcification within the medial layer of the arterial vascular wall (Monckeberg’s sclerosis).
There are two types of neuroarthropathies in DM: atrophic and hypertrophic (Charcot’s joint). Atrophic neuroarthropathy is characterized by osteoporosis, bone resorption, and dislocation. In contrast, Charcot’s joint is characterized by the 5Ds: distention, dislocation, disorganization, debris, and increased bone density. In the absence of diabetes, atrophic neuroarthropathy is commonly caused by syrinx in the cervical spine, while Charcot’s joint is commonly caused by neurosyphilis of the posterior columns of the spinal cord (tabes dorsalis). A syrinx is also the commonest cause of Charcot’s joint of the shoulder.
Diabetic peripheral neuropathy affects 10–15 % of patients, and it can be diffuse or focal. The diffuse form presents in the form of bilateral, symmetrical denervation and sensory deficits of the hands and feet (glove and stocking phenomenon). In contrast, the focal form presents in the form of “mononeuritis,” commonly affecting the cranial nerves CN III, CN IV, CN VI, and CN VII. Involvement of both sympathetic and sensory fibers leads to mechanical overuse, loss of the protective joint pain, proprioceptive sensation, and active hyperemia due to loss of vasoconstrictive neural impulses, which all result in atrophic neuroarthropathy. In contrast, sensory fiber denervation in the absence of sympathetic fiber involvement results in the development of Charcot’s joint. The atrophic joint tends to involve the forefoot, while Charcot’s joint tends to affect the mid- or hindfoot.
Diabetic lumbosacral radiculoplexus neuropathy (DLRPN), also known as Bruns–Garland syndrome, is an uncommon condition, characterized by asymmetric lower extremity pain, weakness, and muscle atrophy commonly affecting the thigh muscles. The mechanism of injury is thought to be a result of microvasculitis and resultant ischemic injury to the sacral plexus and/or peripheral nerves. Patients with DLRPN are commonly between 46 and 71 years of age, often presenting with acute or subacute onset of severe asymmetric lower limb pain and paresthesia involving the anterolateral thigh region. The pain is described as aching and burning and tends to be worse at night or in contact with cloths or bed sheets (contact allodynia). DLRPN pain is usually followed by limb weakness, evolving over weeks or months, and commonly affects the quadriceps and iliopsoas muscles. Wasting of the quadriceps muscle and absence or reduction in the knee jerk reflex are classic features. DLRPN is commonly preceded by unintentional weight loss. Laboratory findings in DLRPN include high erythrocytes sedimentation rate, occasional positive rheumatoid factor (RF) and antinuclear antibody (ANA), and elevated cerebrospinal fluid protein content.
Osteomyelitis occurs in up to 90 % of cases in the diabetic foot, due to neurotropic pedal ulcers. Diabetic ulcers tend to occur at the sites of pressure over bony or joint protuberance (e.g., metatarsal heads or the calcaneus).
The diabetic foot can be rarely associated with tarsal tunnel syndrome. Tarsal tunnel syndrome is a condition characterized by entrapment of the posterior tibial nerve as it passes beneath the flexor retinaculum. The condition is analogous to carpal tunnel syndrome in the wrists. Patients often present with a burning sensation and paresthesia in the toes, sole of the foot, or medial heel, aggravated by weight bearing.
Uncommonly, Freiberg’s disease may arise in patients with diabetic foot. Freiberg’s disease is a disease characterized by infarction of the metatarsal heads. The disease typically develops 3–4 times more frequently in women than men, during late childhood or adolescence. Patients present clinically in the acute phase with local foot pain with tenderness, confined to the area of the metatarsal heads. In the chronic phase, which is characterized by osteonecrosis and repair, patients are typically asymptomatic.
Signs on Plain Radiographs
Charcot’s joint is destruction of the affected with sclerosis (increased bone density), osteophytes (debris), dislocation, and destruction (Fig. 10.1.2).

Fig. 10.1.2
Anteroposterior plain knee radiograph of a patient with sever Charcot’s knee joint demonstrates disorganization, debris, and increased bone density
Calcified vessels may be seen as radio-opaque tubular structures.
An atrophic joint often shows osteoporosis with resorption of the metatarsal distal ends resulting in “pencil and cup” or “sucked candy stick” deformities, similar to those seen in leprosy.
Osteomyelitis is seen as cortical bone destruction with a moth-eaten appearance of the affected bone (Figs. 10.1.1 and 10.1.3).

Fig. 10.1.3
Plain foot radiographs of a patient with diabetic foot show acute osteomyelitis. In (a), the patient was investigated for a pain in the fifth toe, which shows mild osteoporosis compared to the rest of the metatarsals (note the third toe amputation). After 3 months (b), the patient showed moth-eaten osteomyelitis bone destruction of the fifth metatarsal bone, with complete cortical destruction
Lisfranc fracture is a clinical condition where the entire forefoot is displaced laterally. Lisfranc fracture is diagnosed radiographically when the second metatarsal bone is displaced laterally >2 mm from its articulation with the intermediate cuneiform bone (Fig. 10.1.4).

Fig. 10.1.4
Plain radiograph of the forefoot show Lisfranc fracture, with lateral displacement of the metatarsals (arrowhead)
Charcot’s joint of the hip can result in osteolysis of the acetabulum with loss of its boarders (wandering acetabulum) and hypertrophic sclerosis of the femoral head resulting in a “drumstick” appearance.
The talonavicular joint is a preferred site for Charcot’s joint in the hindfoot.
Signs on MRI
In DLRPN, the scan show enhancement of the lumbosacral nerve roots and plexus after contrast injection.
In Freiberg’s infarction, the metatarsal head shows low T1 signal intensity and high T2 signal intensity, with contrast enhancement (Fig. 10.1.5).

Fig. 10.1.5
Plain foot radiograph (a), T1W (b), and sagittal short tau inversion recovery (STIR) (c) foot MRI of a patient show the signs of Freiberg’s infarction. In (a), there is mild flattening and sclerosis of the second metatarsal head (arrowhead). Later, the patient underwent a foot MRI that confirmed bone infarction of the second metatarsal head seen as low T1 signal intensity in (b) and high signal intensity in (c). The MRI shows also fracture of the third metatarsal neck (arrows), which was not well appreciated in the plain radiograph (a)
Diabetic Myonecrosis
Diabetic myonecrosis is a rare complication of diabetes, characterized by muscle infarction. Most patient affected with diabetic myonecrosis are patients with type 1 DM (74 %) and type 2 DM (26 %) or patients with prolonged poorly controlled diabetes. Diabetic myonecrosis occurs in association with diabetic retinopathy (60 %), nephropathy (80 %), or neuropathy (64 %). It almost always occurs in the lower extremities and often affects the quadriceps muscles.
Patients with diabetic myonecrosis commonly present with a painful limb, swelling, and resting pain that is aggravated by walking. If one limb is affected by diabetic myonecrosis, the contralateral limb may be involved up to 2 years after the initial manifestation.
The main differential diagnosis of diabetic myonecrosis includes deep venous thrombosis (DVT) and pyomyositis.
DVT can be ruled out by Doppler sonography. Pyomyositis is a severe muscle infection with formation of an intramuscular abscess. In 90 % of cases, it is caused by Staphylococcus. In contrast, diabetic myonecrosis does not show positive culture of Staphylococcus, because it is mostly caused by ischemia and infarction rather than infection.
Signs on MRI
The affected muscle shows extensive edema and swelling, with high signal intensity on T2W images involving the muscle and the subcutaneous tissues.
Diabetic Skin Changes and Infections
Diabetic hand lesions are not as common as diabetic foot lesions, perhaps due to the stress load on the feet compared to the hands. The main lesions of the hands in diabetes are related to dermatological diseases rather than neuro-osteopathic diseases such as those of the feet.
Diabetic dermopathy is characterized by the formation of multiple skin thickening on the back of the fingers (finger pebbles), scleroderma-like skin and stiff joints of the fingers and dorsum of the hand, and brown atrophic macules over the shin. Acanthosis nigricans is hyperpigmentation and velvety brown thickening of the major skin flexures, which is often seen with type 2 DM and obese patients.
Diabetic hand syndrome refers to a condition of neuropathy denervation of the hand. It is characterized by intrinsic wasting of the hand muscles and atrophy of the palmar tissues, with flexion contractures of the fingers that may mimic Dupuytren’s contracture. Patients with diabetic hand syndrome often complain of carpal tunnel syndrome, with paresthesia in the palmar distribution of the median nerve (the first three fingers) and positive Tinel’s sign (pain and paresthesia initiated in the palmar sensory distribution of the median nerve by tapping over the palmar aspect of the wrist). Moreover, sever neuropathic denervation may lead to Sudeck’s atrophy (shoulder–hand disease). Sudeck’s atrophy is a disease characterized by osteoporosis and swelling in one limb, especially the ankles, wrists, and elbows, after a minor trauma. It results from abnormal sympathetic innervations and secondary vascular changes after minor trauma and typically affects the distal part of a limb below the trauma.
Tropical diabetic hand syndrome, a terminology used to describe a specific infection of the hands in diabetics, usually occurs in tropical areas and is characterized by progressive synergistic gangrene (Meleney’s gangrene) of the hand following minor trauma. The cause of this syndrome is a progressively severe form of cellulitis caused by multibacterial infection, usually after a history of minor trauma or a scratch (Fig. 10.1.6).


Fig. 10.1.6
An illustration demonstrates severe synergistic gangrene of tropical diabetic hand syndrome
Necrobiosis lipoidica diabeticorum (NLD) is a rare, degenerative, granulomatous skin disease that often affects the lower extremities in diabetic patients (0.3 % of diabetics). Lesions are red papules or oval plaques that grow peripherally and become atrophic and yellowish at the center, with elevated and erythematous edges (Fig. 10.1.7). With time, these lesions become more brownish-yellow, telangiectatic, and porcelain-like. In most cases they are bilateral. Ulceration, the most common complication of NLD (35 %), usually arises after a minor trauma. Lesions in NLD are granulomatous, mainly affecting the subcutaneous tissues and the dermis, and the epidermis is often normal or atrophic. NLD is classically found in young Caucasian diabetic patients, with female predominance (80 %); however, it may occur also with sarcoidosis, rheumatoid arthritis, and inflammatory bowel disease.


Fig. 10.1.7
An illustration demonstrates necrobiosis lipoidica diabeticorum lesions on both shins
When normal skin is stroked with a dull object, it rises and swells to assume the shape of the stroke, due to edema and local erythema. In rare situations, exaggeration of this response may be seen in diabetic patients, a condition known as dermatographism (mechanical urticaria). Skin stroke erythema in normal skin develops and subsides in less than 5–10 min, whereas in dermatographism, it can last up to 30 min.
Fournier’s gangrene, also known as necrotizing fasciitis of the scrotum, is a medical emergency that is characterized by rapidly progressing gangrene of the penis and scrotum, usually in diabetic males aged 50–70 years. Fournier’s gangrene is commonly seen after perineal trauma, urinary tract infection, or urological surgical procedures. Fournier’s gangrene is initiated by perianal, perirectal, and ischiorectal abscesses, fissures, or urinary extravasation. Systemic findings include leukocytosis, fever, hypoglycemia, tachycardia, and dehydration.
The skin of the back of the neck is surrounded by tough deep fascia that attaches to the epidermis layer by fibrous bands, creating separated compartments. In diabetics, subcutaneous infection on the back of the neck is localized by these fibrous bands laterally and inferiorly, forcing the abscess to spread to the surface via a sinus. Multiple intercommunicating abscesses that open into the surface via multiple sinuses in diabetic patient is a special type of abscess called “carbuncle.”
Signs on Plain Radiographs
Changes in the hand due to Sudeck’s atrophy are typically seen as severe osteoporosis, which occurs at the ends of all the phalanges and up to 70 % of metatarsal heads (Fig. 10.1.8). Pseudoperiostitis may be seen as striation of the cortices due to new bone formation. Severe subluxation of the phalangeal joints may occur later in the course of the disease.


Fig. 10.1.8
Plain hand radiograph of a patient with Sudeck’s atrophy shows marked osteoporosis of the hand that is localized to the phalanges and the metatarsal heads (arrowheads)
Signs on US
Carpal tunnel syndrome can be diagnosed with wrist ultrasound by identifying the nerve below the flexor retinaculum. Diagnosis of nerve entrapment is achieved when the nerve transverse diameter exceeds 10 mm due to edema or when the nerve fails to return to its normal position when performing the flexion pinch maneuver (Fig. 10.1.9).

Fig. 10.1.9
Median nerve ultrasound in a healthy volunteer shows the normal median nerve (arrowhead) seen below the flexor retinaculum (arrow) as a hypoechoic structure in (a) and (b). The median nerve transverse diameter was 4 mm. In the flexion pinch maneuver, the patient is asked to flex his wrist, forcefully oppose the thumb to the index finger, hold the position for 3–5 s, and then release. In this maneuver, the median nerve moves in a sagittal motion deep into the carpal tunnel (arrowhead in b) and then returns to its normal position. Failure of the nerve to return to its normal position or to move deep into the carpal tunnel with this maneuver is a sign of entrapment
Fournier’s gangrene is characterized by thickening of the scrotal skin, with gas formation within the subcutaneous skin, seen as hyperechoic foci surrounded by dirty shadowing.
Signs on CT
Fournier’s gangrene is seen as thickened scrotal and/or penile skin with hypodense soft-tissue fluid collection surrounded by rim contrast enhancement (abscess). Air within the mass and the subcutaneous tissues is a typical sign of necrotizing fasciitis (Fig. 10.1.10).


Fig. 10.1.10
Axial postcontrast CT of the scrotum and the upper thighs shows scrotal abscess with areas of ring contrast enhancement (arrows) and gas formation (arrowhead); a radiological stigma of Fournier’s gangrene
Signs on MRI
In patients with carpal tunnel syndrome, there is a typical flattening of the median nerve, with high signal intensity in T2W images with contrast enhancement, due to inflammation.
The Role of Doppler Sonography in DM
Doppler sonography is used to detect stenosis within the arterial system of the lower extremities. Arteriosclerosis is the most common cause of arterial stenosis with the formation of atheromas and calcium plaques within the arterial walls. Analysis of the Doppler wave spectrum is essential to detect the hemodynamic abnormalities of circulation in the lower limbs. Different spectral waves are observed, according to the degree of stenosis.
Signs of Peripheral Vascular Disease on Doppler Scan (Can Be Detected Even Before the Appearance of Clinical Symptoms)
Medial arterial wall calcification with acoustic shadowing string of beads sign (Fig. 10.1.11).

Fig. 10.1.11
Sagittal ultrasound image of the superficial femoral artery in a diabetic shows multiple dense calcifications of the arterial wall (arrowheads)
Increased diastolic flow with reduced resistance index (RI) in the spectral flow analysis (Fig. 10.1.12). The increase in diastolic flow is due to arteriovenous shunting.

Fig. 10.1.12
Sagittal ultrasound image of the superficial femoral artery in a patient with peripheral vascular disease due to diabetes mellitus (DM) shows monophasic arterial spectral wave with increased diastolic flow (arrowhead)
Spectral flow abnormalities.
Spectral Flow Abnormalities on Doppler Scan of the Lower Limbs
The normal arterial spectrum is triphasic, with peak systolic velocity (PSV) ~120 cm/s.
0–50 % stenosis: shows triphasic or biphasic arterial spectrum (due to loss of the reversal flow pattern), with PSV <180 cm/s.
50–75 % stenosis: shows biphasic or monophasic arterial spectrum, with PSV >180 cm/s (Fig. 10.1.13).

Fig. 10.1.13
Sagittal ultrasound image of the superficial femoral artery in a patient with peripheral vascular disease due to DM shows monophasic arterial spectral wave with PSV >250 cm/s (arrowhead), representing >75–99 % arterial stenosis
75–99 % stenosis (high grade): shows biphasic or monophasic arterial spectrum, with PSV >250 cm/s.
As the stenosis becomes generalized and affects a long segment of the artery, the flow spectrum becomes biphasic or monophasic, the acceleration upstroke is reduced, and the systolic peak becomes rounded (Fig. 10.1.14).

Fig. 10.1.14
Sagittal ultrasound image of the superficial femoral artery in a patient with peripheral vascular disease due to DM shows monophasic arterial spectral wave with PSV <50 cm/s (arrowhead). As mentioned earlier, the normal peripheral arterial spectral wave for the superficial femoral artery is triphasic, with PSV <120 cm/s
Signs of Diabetic Nephropathy on Doppler Scan
Increased renal length and parenchymal thickness due to glomerular hyperfiltration. The normal kidney size is 10–12 cm in the longitudinal diameter and 4–6 cm in the transverse diameter; the normal renal parenchymal thickness is 1.6 cm.
RI of arcuate arteries is >0.7.
In advanced renal disease, there is increased echogenicity of the renal cortex, with reduction of its thickness.
The Role of MRI in DM
MRI is a powerful tool to detect early bone changes that may not be seen on plain radiographs or evoke complaints – unless they are severe and destructive. Contrast-enhanced studies should be done to detect signs of soft-tissue inflammation, abscess formation, sinus tract detection, and devitalization.
Signs of Diabetic Foot on MRI
A callus is detected on MRI as a soft-tissue area of low T1 signal intensity with intense contrast enhancement after contrast injection. It is typically found at the first and fifth metatarsal heads, the malleoli, and the calcaneus (Fig. 10.1.15).

Fig. 10.1.15
Sagittal T1W (a) and STIR (b) ankle MR illustrations demonstrate callus seen as an area of soft tissue with low T1 signal intensity in (a) and with high T2 signal intensity in (b) (arrowheads)
Devitalization is an area of soft tissue that is devoid of vascular supply (tissue infarction). It is detected on MRI as a soft-tissue area with low T1 and low T2 signal intensities, which shows no contrast enhancement after contrast injection (like any other tissue infarction in the body). It is important to inform the surgeon about devitalization areas for tissue debridement planning.
An ulcer is detected as an area of skin and soft-tissue defect, with low signal intensity on T1W images and intense enhancement after contrast administration.
Cellulitis is an area of soft-tissue inflammation and is detected on MRI as an ill-defined area of soft tissue with low T1 and high T2 signal intensities, with ill-defined enhancement after contrast administration (Fig. 10.1.16).

Fig. 10.1.16
Sagittal T1W (a) and STIR (b) ankle MRI show areas of low T1 and high T2 signal intensity lesions confined to the skin and the subcutaneous tissue, without signs of bone marrow edema or joint effusion (arrowheads), representing cellulites
An abscess is a localized area of pus collection and typically detected as acystic area of low T1 and high T2 signal intensities, with ring enhancement after contrast administration (Fig. 10.1.17).

Fig. 10.1.17
Sagittal T1W postcontrast (a) and STIR (b) ankle MR illustrations demonstrate abscess formation, seen as a cystic area surrounded by rim contrast enhancement in (a) and seen as an area of cystic fluid collection in (b)
Reactive bone marrow is detected as a normal (isointense) signal intensity of the bone marrow on T1W images, with high signal intensity on T2W images (Fig. 10.1.18).

Fig. 10.1.18
Sagittal T1W (a) and STIR (b) ankle MR illustrations demonstrate reactive bone edema affecting the posterior third of the calcaneus
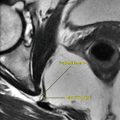
Osteomyelitis is inflammation of the bone and the bone marrow. It is detected on MR as areas of cortical bone defect characterized by the following characteristics: it diffuses bone marrow edema, may show sequestrum, shows no bone deformities (unless complicated by neuropathic joint), usually underlies an ulcer (e.g., metatarsal heads), and may show sinus formation into the skin surface, and the soft tissue around it is usually inflamed and shows marked contrast enhancement (Fig. 10.1.19). Signs of periostitis may be found, which is seen as linear contrast enhancement surrounding the outer cortical margin. An intraosseous abscess may occur in subacute osteomyelitis (Brodie’s abscess), which is characterized by the penumbra sign. The penumbra sign is detected on MRI as a discrete zone of peripheral T2 hyperintensity signal surrounding a high T2 signal intensity intraosseous abscess (Fig. 10.1.19). Osteomyelitis is classically located in a single focus. However, multiple lesions may be seen in 20 % of cases.

Fig. 10.1.19
Sagittal T1W postcontrast (a) and STIR (b) ankle MR illustrations show signs of osteomyelitis. Notice the calcaneal ulcer with edema (white arrowhead), the penumbra sign (black arrowhead), sinus tract from the osteomyelitis spreading infection to the nearby soft tissues (arrow), and signs of periostitis seen as linear high signal intensities located around the cortex of the calcaneus (hollow arrowhead)
Septic arthritis is inflammation of a joint due to infection. It is detected on MRI as high T2 signal intensity within a joint and its surrounded soft tissue, with signs of joint effusion and cartilage destruction. There is intense enhancement of the joint and its surrounding soft tissue after contrast administration (Fig. 10.1.20).

Fig. 10.1.20
Sagittal STIR ankle MR illustration demonstrates talonavicular joint septic arthritis, seen as bone marrow edema affecting the articular bones with joint effusion
A foreign body is detected on MRI as an object of low T2 signal intensity, surrounded by a high-intensity signal in the soft tissues on T2W images (due to edema around the foreign body) (Fig. 10.1.21).

Fig. 10.1.21
Sagittal STIR ankle MR illustration demonstrates a foreign body surrounded by tissue edema located within the infracalcaneal soft-tissue region
Neuroarthropathic joint (Charcot’s joint) has the same presentation and signal intensities as osteomyelitis, with destruction of the subchondral cortices. Neuropathic joint is characterized by midfoot predominance, subchondral cyst formation, normal surrounding tissue with intact overlying skin, juxta-articular edema, signs of joint disorganization and deformity (5Ds), and no signs of fluid collection or abscess (Fig. 10.1.22). Osteomyelitis, in contrast to neuropathic joint, predominates in pressure areas such as the metatarsal heads in the forefoot and the calcaneus in the hindfoot. The only common location for osteomyelitis in the midfoot is in the cuboid bone, which occurs in severe midfoot neuropathic joint. However, bone biopsy remains the definite diagnostic method to differentiate osteomyelitis from neuropathic joints in diabetics.

Fig. 10.1.22
Sagittal STIR ankle MR illustration demonstrates talonavicular Charcot’s joint. Notice the midfoot location, the joint deformity (arrow), the subchondral cysts (arrowhead), and the mild joint effusion due to reactive inflammation
Tenosynovitis is detected as normal tendon size, surrounded by high T2 fluid-signal intensity on T2W images.
Calcaneal insufficiency avulsion fracture is an extra-articular fracture affecting the posterior third of the calcaneus (Fig. 10.1.23). It is seen almost exclusively in diabetics. Sinus tract is detected as a hypodense line extending from an area of bone destruction to the adjacent soft tissues (Fig. 10.1.19). It is best detected on postcontrast fast-suppressed T1W images.

Fig. 10.1.23
Sagittal T1W ankle MR illustration demonstrates avulsion fracture of the posterior third of the calcaneus (arrowheads)
Differential Diagnoses and Related Diseases
Congenital insensitivity to pain (CIPA): CIPA, also referred to as hereditary sensory and autonomic neuropathy type IV, is a rare disorder characterized by the inability to perceive pain stimuli due to peripheral autonomic nervous system demyelination and reduced fiber caliber. CIPA patients respond to normal pain stimuli but not to painful stimuli. Early symptoms include decreased sweating (anhidrosis), which causes episodes with extreme hyperpyrexia, multiple healed tongue bites since infancy, and multiple missing teeth due to auto-extraction (50 % of cases). Characteristically, CIPA patients present with multiple bony features at varying stages of the healing process. Interestingly, patients with CIPA develop aseptic necrosis and osteochondritis in the juxta-articular regions of the weight-bearing long bones (hip, knees, and ankles). Joint radiographs of CIPA patients show changes similar to those of chronic Charcot’s joint and osteomyelitis.
Further Reading
Abdel-Hafez HZ, et al. Congenital insensitivity to pain with anhidrosis (CIPA). Egypt Dermatol Online J. 2007;3(1):5.
Beltran J, et al. The diabetic foot: magnetic resonance imaging evaluation. Skeletal Radiol. 1990;19:37–41.
Bhanushali MJ, et al. Diabetic and non-diabetic lumbosacral radiculoplexus neuropathy. Neurol India. 2008;56(4):420–5.
Bhute D, et al. Dermatographism. Indian J Dermatol Venerol Leprol. 2008;74:177–9.
Biswal N, et al. Congenital indifference to pain. Indian J Pediatr. 1988;65:755–69.
Chantelau E, et al. “Silent” bone stress injuries in the feet of diabetic patients with polyneuropathy: a report on 12 cases. Arch Orthop Trauma Surg. 2007;127:171–7.
Chatha DS, et al. MR imaging of the diabetic foot: diagnostic challenges. Radiol Clin North Am. 2005;43:747–59.
Chuter V, et al. Limited joint mobility and plantar fascia function in Charcot’s neuroarthropathy. Diabet Med. 2001;18:558–61.
Erickson SJ, et al. MR imaging of the tarsal tunnel syndrome and related spaces: normal and abnormal findings with anatomic correlation. AJR Am J Roentgenol. 1990;155:323–8.
Gefen A, et al. Integration of plantar soft tissue stiffness measurements in routine MRI of the diabetic foot. Clin Biomech. 2001;16:921–5.
Glauser SR, et al. Diabetic muscle infarction: a rare complication of advanced diabetes mellitus. Emerg Radiol. 2008;15:61–5.
Gold RH, et al. Imaging the diabetic foot. Skeletal Radiol. 1995;24:563–71.
Jung Y, et al. Diabetic hand syndrome. Metabolism. 1971;20(11):1008–15.
Marcus CD, et al. MR imaging of osteomyelitis and neuropathic osteoarthropathy in the feet of diabetics. Radiographics. 1996;16:1337–48.
McGuinness M, et al. Necrobiosis Lipoidica diabeticorum. Foot. 1997;7:47–51.
Naderi ASA, et al. Diabetic muscle necrosis. J Diabet Complications. 2008;22:150–2.
Nguyen VD, et al. Freiberg’s disease in diabetes mellitus. Skeletal Radiol. 1991;20:425–8.
Nguyen K, et al. Necrobiosis Lipoidica diabeticorum treated with chloroquine. J Am Acad Dermatol. 2002;46:S34–6.
Peyri J, et al. Necrobiosis lipoidica. Semin Cutan Med Surg 2007;26(2):87–9.
Piedra T, et al. Fournier’s gangrene: a radiologic emergency. Abdom Imaging. 2006;31:500–2.
Purewal TS. Charcot’s diabetic neuroarthropathy: pathogenesis, diagnosis and management. Pract Diab Int. 1996;13(3):88–91.
Puttemans T, et al. Diabetes: the use of color Doppler sonography for the assessment of vascular complications. Eur J Ultrasound. 1998;7:15–22.
Reinhardt K. The radiological residua of healed diabetic arthropathies. Skeletal Radiol. 1981;7:167–72.
Singson RD, et al. Postamputation neuromas and other symptomatic stump abnormalities: detection with CT. Radiology. 1987;162:743–5.
Singson RD, et al. Postamputation neuromas. Skeletal Radiol. 1990;19:259–62.
Tan PL, et al. MRI of the diabetic foot: differentiation of infection from neuropathic change. Br J Radiol. 2007;80:939–48.
Tiwari S, et al. Tropical diabetic hand syndrome. Int J Diab Dev Ctries. 2008;28(4):130–1.
10.2 Diabetic Brain and Nervous System
In advanced stages, diabetes mellitus (DM) can affect the brain, due to microangiopathy and prolonged exposure to hypoglycemia. Over the past decade, many researches have evaluated the anatomical and functional status of the brain in diabetics compared to the normal population. This topic presents the most common, well-documented brain changes in diabetics, as reported in the medical and radiological literature.
DM type 1 can be associated (rarely) with chorea-ballismus episodes due to nonketotic hyperglycemia (NKH). The cause of these chorea-ballismus episodes is unknown, but it is believed that they are vascular in origin.
Chorea is defined as involuntary, continuous, random, fast, jerking, dance-like movements in the distal parts of the limbs. Ballismus shows a picture similar to chorea, but the movements are more irregular, of large amplitude, and violent, affecting the proximal portion of limbs.
Nonketotic hyperglycemia (NKH) is a severe form of hyperglycemia with hyperosmolarity and intracellular dehydration, with little or no ketoacidosis. It is typically observed in diabetic patients >50 years of age. NKH is characterized by partial insulin deficiency with enough insulin to inhibit ketoacidosis but not enough to transport glucose into the cells. Hyperglycemia causes an osmotic diuresis, with progressive dehydration, resulting in NKH. Up to 40 % of patients with NKH develop seizures beside the chorea-ballismus episodes.
The incidence of stroke is six times higher in patients with DM than in nondiabetics. This high stroke risk can be explained by the high incidence of atherosclerosis of the internal carotid artery in diabetics.
Many researchers reported high cerebral brain atrophy among long-term diabetics. Patients with DM type 2 were found to have an increased risk of Alzheimer’s disease (AD) and vascular dementia. AD in diabetics is believed to be due to the increase in advanced glycation end products, which increase aggregation of proteins involved in AD development. Furthermore, dysfunction of insulin signaling in the brain has been implicated in the pathogenesis of AD. Subcortical arteriosclerosis encephalopathy can develop in diabetics due to brain vessel atherosclerosis.
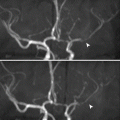
Cranial nerve involvement in DM is a rare complication. A single cranial nerve (diabetic mononeuritis) or multiple cranial nerves (mononeuritis multiplex) can be involved. DM classically affects the cranial nerves CN III, CN IV, CN VI, and CN VII. Cranial nerve involvement in diabetes is thought to be a result of microvasculitis and resultant ischemic injury to the nerves.
Patients with diabetic ketoacidosis (DKA) can develop subclinical cerebral edema for unknown reasons. The brain edema can start before or after treatment initiation. Patients with DM type 1 are most commonly affected, and it occurs in > 1 % of cases. Typically, patients present with severe headaches that can progress (rarely) into brain herniation. Other manifestations of symptomatic brain edema due to DKA include a drop in heart rate, altered mental status that ranges from dizziness to coma, and increased blood pressure.
Signs on CT and MRI
Stroke is seen as a hypodense area on CT or a hyperintense area on T2W MR images. Focal neurological deficits and the clinical picture suggest the diagnosis.
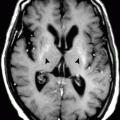
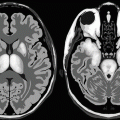
In NKH–ballismus episode, CT scan of the basal ganglia (caudate and putamen) is hyperdense compared to the rest of the brain parenchyma (Fig. 10.2.1). Normal basal ganglia attenuation is between 33 and 36 HU. In diabetic chorea, the basal ganglia show attenuation between 40 and 51 HU. This finding is believed to be caused by multiple petechial hemorrhages within the basal ganglia. On MRI, the basal ganglia show hyperintense signal intensity on both T1W and T2W images (Fig. 10.2.1). This sign is not specific and can be observed in cases of hepatic encephalopathy, carbon monoxide toxicity, Wilson disease, and neurofibromatosis.

Fig. 10.2.1
Axial nonenhanced brain CT (a) and MR (b) illustrations demonstrate high-density basal ganglia (a) and high-intensity signal of the basal ganglia (b), which is a sign detected in patients with ballismus episodes and in patients with nonketotic hyperglycemia
Generalized brain atrophy is seen in diabetics with signs of dementia. The hippocampus and the amygdale volume are reduced in diabetics who develop signs of dementia.
Signs of subcortical arteriosclerosis encephalopathy may be seen when the clinical status of the patient suggests dementia.
Diabetic mononeuritis is detected as enhancement of the affected cranial nerve after contrast injection ipsilateral to the site of cranial nerve clinical deficits. Multiple cranial nerve enhancements are seen in mononeuritis multiplex.
Diabetic ketoacidosis brain edema often shows signs of loss of gray-white matter differentiation, effacement of the sulci, and decrease in ventricular size.
Further Reading
Araki Y, et al. MRI of the brain in diabetes mellitus. Neuroradiology. 1994;36:101–31.
Brands AMA, et al. Cognitive functioning and brain MRI in patients with type 1 and type 2 diabetes mellitus: a comparative study. Dement Geriatr Cogn Disord. 2007;23:343–50.
Harten BV, et al. Brain imaging in patients with diabetes. A systematic review. Diabetes Care. 2006;29(11):2539–46.
Kelkar P, et al. Mononeuritis multiplex in diabetes mellitus: evidence for underlying immune pathogenesis. J Neurol Neurosurg Psychiatry. 2003;74:803–6.
Lai PH, et al. Chorea-ballismus with nonketotic hyperglycemia in primary diabetes mellitus. AJNR Am J Neuroradiol. 1996;17:1057–64.
Lavin PJM. Hyperglycemic hemianopia: a reversible complication of non-ketotic hyperglycemia. Neurology. 2005;65: 616–9; den Heijer T, et al. Type 2 diabetes and atrophy of medial temporal lobe structures on brain MRI. Diabetologia. 2003;46:1604–10.
Pacheco E, et al. Pathophysiology and computed tomography findings in a case of diabetic ketoacidosis. Int Pediatr. 1999;14(2):118–20.
Witzke KA, et al. Diabetic neuropathy in older adults. Rev Endocr Metab Disord. 2005;6:117–27.
10.3 Diabetic Syndromes
Diabetic syndromes are a group of diseases characterized by the development of diabetes mellitus (DM) in childhood. Most of these diseases are originally syndromes, with DM constituting a major manifestation of these syndromes. This topic describes the most common pediatric syndromes associated with DM.
Alström Syndrome
Alström syndrome (AS) is a very rare genetic disease, characterized by infantile dilated cardiomyopathy, diabetes mellitus, pigmentary retinal dystrophy causing blindness, sensorineural hearing loss, and obesity.
The disease has an autosomal recessive mode of inheritance, and it is linked to mutation in the short arm of chromosome 2. Dilated cardiomyopathy is the earliest manifestation of this syndrome and classically starts in the third to fourth week of life. Blindness and sensorineural hearing loss in an obese child should trigger the suspicion of AS.
Diagnostic and key features of AS include:
Ophthalmologic Clinical Findings
Atypical pigmentary retinopathy without classical bone spicules is a constant finding (diagnostic criterion).
Nystagmus usually appears during the first 1–2 years of life.
Visual deterioration occurs during the first decade of life.
Loss of the papillary reactions appears during the second decade of life.
Auditory Clinical Findings
Progressive, bilateral sensorineural hearing loss is found within the first decade of life (diagnostic criterion).
Metabolic and General Clinical Findings
Obesity with hypertriglyceridemia from birth (diagnostic criterion).
Hyperinsulinemia and noninsulin-dependent DM (diagnostic criterion).
Hepatic failure and hypogonadism are found occasionally.
Renal Clinical Findings
Renal deterioration is a constant finding, and it is age related, often starting within the second decade of life.
Dermatological Clinical Findings
Areas of hyperpigmentation and papillary hypertrophy on the neck and flexor creases (acanthosis nigricans) may be found occasionally.
Signs on Chest Radiographs
Increased cardiaothoracic ratio and signs of dilated cardiomyopathy can be seen in advanced stages.
Signs on US and CT
Multiple hyperechoic or hypodense liver lesions may be found with heterogeneous contrast enhancement. The lesions are usually due to hepatocellular adenoma with pericellular fibrosis.
Bardet–Biedl Syndrome
Bardet–Biedl syndrome (BBS) is a rare, genetically heterogeneous disease, characterized by noninsulin-dependent DM in adulthood, ocular abnormalities, mental retardation, obesity, polydactyly, and renal dysfunction.
BBS has an autosomal recessive mode of inheritance, with prevalence of 1 in 125,000 live births. Key features of BBS include pigmentary (rod-cone) retinal dystrophy in the second decade of life (95 % of cases), truncal obesity with normal appetite (85 % of cases), genital hypoplasia (74 % of cases), mental retardation (70 % of cases), postaxial polydactyly (80 % of cases), and renal anomalies. Chronic end-stage renal failure is a constant feature (100 % of cases). Renal anomalies are the main cause of mortality in BBS patients.
Signs on IVU
Bilateral small kidney size
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree