Fig. 5.1
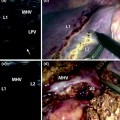
Screen of the ultrasound system regulated by contrast preset. In the upper right corner, MI (yellow arrow) means mechanical index, which corresponds with the power at which the ultrasound waves intercept the microbubbles composing the contrast agent, which should be below 1, in order not to break the microbubbles and providing the contrast effect of real-time enhancement in ultrasound. In the upper left corner, the time (yellow arrow) elapsing from injection of the contrast agent by the anesthesiologist into a peripheral vein
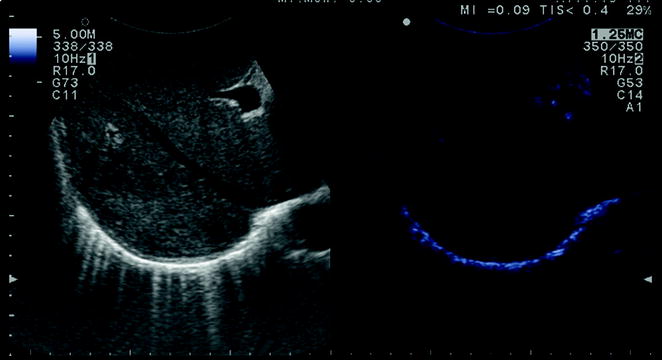
Fig. 5.2
The screen of the ultrasound system can be set to combine on the same image the preset for B-mode (left) and contrast (right)
5.2 Indications
5.2.1 Hepatocellular Carcinoma
As mentioned for HCC, CEIOUS is now used for characterizing new lesions initially detected by IOUS [6]: the rationale is to check the vascular pattern during contrast enhancement of each new lesion. Since, in the case of HCC, it is very important to identify the arterial vascularization, which lasts from 20 to 30 s, each nodule has to be carefully evaluated, and that demands multiple injections in the presence of multiple nodules. This may no longer be necessary with the use of hepato-specific contrast agents (see Chap. 6).
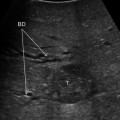
Tumor vascularity is a criterion for differentiating regenerative or dysplastic nodules from the HCC well correlated with the histological evidence of a progressive increase in unpaired arteries from dysplastic to neoplastic nodules in a cirrhotic liver [7]. However, the pattern of vascular enhancement is not sufficient for clearly differentiating malignant from nonmalignant nodules in a cirrhotic liver. Percutaneous contrast-enhanced ultrasound (CE-US) provides a 95 % specific differential diagnosis of focal liver lesions [4]; this rate is, however, referred to another type of lesion if compared to the target of CEIOUS. Intraoperative exploration profits from the higher resolution of US done in direct contact with the liver. Therefore, nodules detected by IOUS are usually smaller than 1 cm: here, vascularity as a criterion for differential diagnosis is less specific. However, some improvements compared with conventional IOUS can be expected. Our preliminary experience showed that CEIOUS can provide remarkable findings, either by the additional information on nodular vascularity in patients with HCC, or by detecting nodules that were not visible in IOUS in patients with CLM [5]. For patients with HCC, we introduced a classification of the pattern of enhancement by CEIOUS of the lesions detected in IOUS from which surgical decision-making can be established (Fig. 5.3) [6]. Briefly, any lesion with a pathologic behavior should appear as hypoechoic in the late phase and with an arterial phase in which it is fully enhanced prior to the remnant liver parenchyma (Fig. 5.4a), or with just inner vessels visualized in it (Fig. 5.4b): this kind of lesions are removed. Those lesions which disappear once the contrast enhances the liver are not considered neoplastic, and those are not removed (Fig. 5.5). With these criteria we obtained a specificity of 69 % by CEIOUS [6]. This value is not very high especially when compared with that reported for CE-US [4]. However, as mentioned above, the small size of the lesions targeted by CEIOUS could explain this discrepancy: for these tiny nodules, there are limits to the use of neo-vascularity as a criterion for differentiating between malignant and benign lesions, which are independent from the method we use. Therefore, CEIOUS can be helpful in a certain percentage of nodules but not in all: in this respect the rate of 69 % of specificity is encouraging as it means that we can provide proper information with this new technique in seven out of ten lesions we detect at the time of laparotomy. For the remaining three, even histology may be lacking. Indeed, we are aware that there is no common agreement between pathologists in East and West on the definition of early HCC and dysplastic lesions [8]. The possible new perspective provided by the new contrast agent, is, as mentioned, still the object of extensive analyses (see Chap. 6).
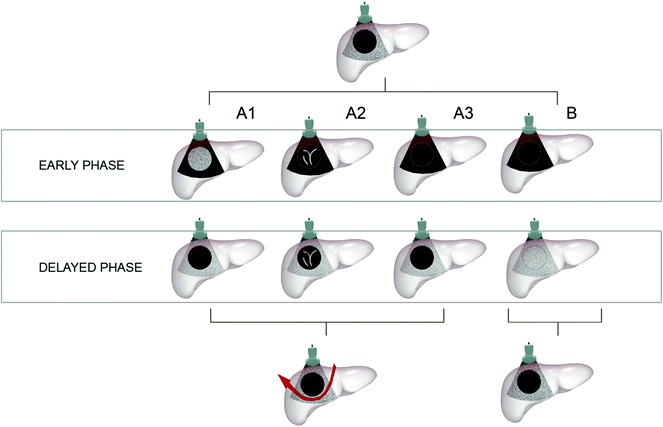
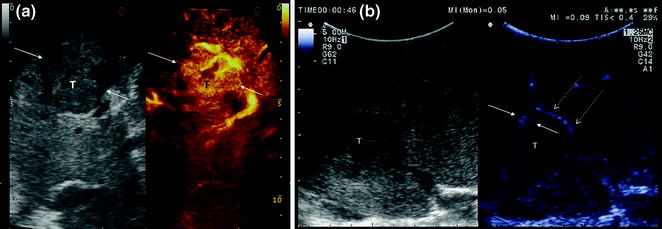
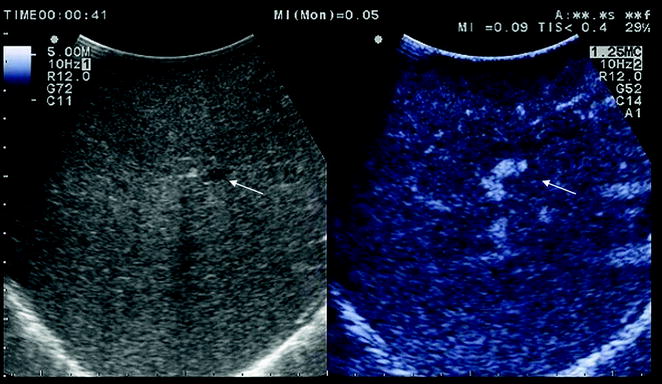

Fig. 5.3
Classification of patterns of enhancement in CEIOUS of those lesions detected in IOUS during surgery for HCC. Lesions having a class A pattern, featured by a hypervascular enhancement in early phase (A1-2), or any hypoechoic pattern in the delayed phases (A1-3), has to be resected; while lesions showing a class B pattern of enhancement are not removed

Fig. 5.4
a At an early stage, the lesion (T) on the right assumes contrast prior to the surrounding tissue showing a nodular arterial enhancement (A1 pattern); b while this lesion is not fully enhanced in the arterial phase but just shows inner vessels (arrows) originating from a perinodular basket of arteries (dashed arrows), and flowing into the nodule itself (A2 pattern)

Fig. 5.5
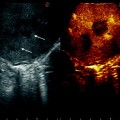
In the delayed phase, the lesion visible on the left in B-mode exploration (arrow) is no longer detectable on the right by CEIOUS (arrow) (B pattern)
5.2.2 Colorectal Liver Metastases
Echogenicity impacts detection of CLM [1], as previously mentioned. Therefore, a modality that would improve lesion visibility, and enhancing detection, was needed: CEIOUS addresses this main goal in the case of CLM. In the 1990s, in half of the patients undergoing surgery for CLM the surgical strategy was modified by IOUS findings [9]. However, more recently, progress in preoperative imaging has reduced this rate: in fact, some authors have recently reported that merely 4 % of operative decision-making has been modified by IOUS [10]. By adding CEIOUS to IOUS exploration, operation decision-making has been affected in 38 % of patients with CLM [11]: if this discrepancy in the rates of modified surgical strategy among series is partially motivated by the different surgical approach, which by latest experience is featured by more parenchymal sparing procedures (see Chap. 7), probably CEIOUS is playing a role too. By using CE-US, CLM has shown a so-called ‘‘black-hole’’ effect (Fig. 5.6): the metastastic nodule in the late phase (2–5 min after injection) remains unenhanced and then becomes black in comparison with the surrounding enhanced liver parenchyma. Therefore, CEIOUS allows better nodule visibility. However, with growing experience, we paradoxically witnessed a decrease in the rate of detectable new lesions from 44–77 % in the first two reports [12, 13] to 17–19 % in the most recent reports [11, 14]. The clinical impact of CEIOUS thus seems to progressively decrease with the improvement of preoperative imaging. However, looking at the number of new lesions detected by IOUS, the latter explanation may not be convincing. Indeed, the 16 % of new lesions detected by IOUS in the first report [12] has been substantially confirmed by more recent experience [11, 14]. Technologically, the improvements in IOUS can explain the still high rate of new lesions detected intraoperatively. However, technological improvements in CEIOUS have been observed in the last years, too, with the introduction of new contrast agents (see Chap. 6




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree