Fig. 1.1
AASLD diagnostic algorithm. Reprint from Bruix J, Sherman M; American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology. 2011 Mar;53(3):1020–2
1.4 Pathological Diagnosis of Dysplasia and Early HCC
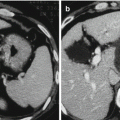
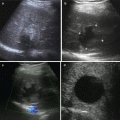
One of the consequences of surveillance programs is the identification of smaller HCCs, as well as dysplastic nodules. The smaller the HCC lesion, the more difficult for us to distinguish malignant from benign nodules. This is true both radiologically and histologically. Recently, a distinction has been made between “very early HCC” [29, 30] and “small” or “progressed” HCC [31, 32]. Early HCC, as defined by Japanese pathologists, is generally hypovascular and has ill-defined margins [9]. Thus, it has a somewhat vague outline on ultrasound and may be hypovascular on CT scanning.
Histologically, there are few unpaired arteries, but the cells show varying grades of dysplasia [33]. There may be invasion of the portal space by hepatocytes, but vessel invasion is absent. These lesions have been called “very early HCC” in the Barcelona Clinic Liver Cancer (BCLC) staging scheme [34]. The pathology of these “very early HCC” lesions has been defined in resected specimens, and therefore, the natural history of these lesions is unclear. However, the presence of small foci of typical HCC within them has been noted, suggesting that these lesions are precursors of typical HCC lesions [29, 31, 32]. The frequency with which these lesions develop typical HCC is unknown either. In contrast, “small” or “progressed” HCC has well-defined margins on ultrasound and exhibits the typical features of well-differentiated HCC on CT and on histology [29, 31, 33]. These lesions often show microvascular invasion, despite their small size [33]. The presence of microvascular invasion suggests that the prognosis of these lesions is less good than for “early HCC” where vascular invasion is rare. However, this has not been proven in clinical studies.
The classification and description of dysplastic nodules and early HCC have been recently revised to harmonize the approaches taken by Western and Japanese pathologists [31]. These studies have been carried out on resected tissue, whereas samples from lesions detected on surveillance usually only have a needle biopsy to evaluate. It is important to understand that rather than being individual discrete states, there is a continuum between HGDN and HCC. This complicates the evaluation of biopsies from small nodules. Patients with liver nodules having a nonspecific vascular profile and negative biopsy should continue to undergo enhanced follow-up as well. There are no data to establish the best follow-up policy so far, but repeated biopsy or follow-up CT/MRI to detect further growth should be considered. There are emerging data indicating that the smaller the lesion, the less likely there is to be microscopic vascular invasion [9].
In addition, smaller lesions are more likely to be associated with treatment that will be curative [30, 35, 36]. Finally, decision analysis also confirms that ideally, for the best outcome, the lesion should be smaller than 2 cm at diagnosis [37]. However, it is equally important not to apply invasive treatment to lesions that do not have any malignant potential and may still regress. This is a fine distinction that is not always possible to make. Another concern about thin needle liver biopsy is the risk of bleeding and needle track seeding. Most studies that report needle track seeding do not specify the lesion size being biopsied [38]. Although the rate of needle track seeding after biopsy of small lesions (<2 cm) has not been accurately measured, it is probably uncommon. The current rate of bleeding from thin needle biopsy of small HCC has not been reported but is probably no different than for biopsy of the liver in general [38, 39].
1.5 Diagnostic Recommendations in AASLD Guideline
1.
Nodules smaller than 1 cm should be followed with ultrasound at intervals from 3 to 6 months. If there has been no growth over a period of up to 2 years, one can revert to routine surveillance.
2.
Nodules larger than 1 cm found on a cirrhotic liver should be investigated further with either four-phase multidetector CT scan or dynamic contrast-enhanced MRI. If the appearances are typical of HCC (i.e., hypervascular in the arterial phase with washout in the portal venous or delayed phase), the lesion should be treated as HCC. If the findings are not characteristic or the vascular profile is not typical, a second contrast-enhanced study with the other imaging modality should be performed, or the lesion should be biopsied.
3.
Biopsies of small lesions should be evaluated by expert pathologists. Tissue that is not clearly HCC should be stained with all the available markers including CD34, CK7, glypican 3, HSP-70, and glutamine synthetase to improve diagnostic accuracy.
4.
If the biopsy is negative for patients with HCC, the lesion should be followed by imaging at 3–6 monthly intervals until the nodule either disappears, enlarges, or displays diagnostic characteristics of HCC. If the lesion enlarges but remains atypical for HCC, a repeat biopsy is recommended.
1.6 EASL Guidelines
The European Association for the Study of the Liver expert panel proposed the following surveillance recall and diagnostic strategy (Fig. 1.2) [40]. In nodules smaller than 1 cm, which are malignant in less than 50 % of cases, reliable HCC diagnosis is difficult. Thus, close follow-up is recommended. In nodules of 1–2 cm, HCC diagnosis requires positive cytohistology. However, there is a 30–40 % false-negative rate with fine-needle biopsy [41]. A negative result does not rule out malignant disease. Noninvasive diagnostic criteria to be applied solely in patients with cirrhosis and tumors larger than 2 cm were proposed. HCC diagnosis is established by the concomitant finding of two imaging techniques, showing a nodule larger than 2 cm with arterial hypervascularization, or by one positive imaging technique, showing hypervascularization associated with alpha-fetoprotein concentration higher than 400 ug/L [27]. The accuracy of imaging techniques is rapidly evolving. Ultrasonography plays a key part in the detection of HCC, but its sensitivity to detect additional small nodules is low [42]. New contrast agents might increase the accuracy of this technique and might be relevant to assess treatment response [43]. The best techniques are helical CT and MRI with contrast enhancement, which have an accuracy exceeding 80 % [44]. CT and MRI sensitivity decreases when assessing the extent of the disease. In a comparison of CT and MRI with pathological examination of explanted livers, MRI angiography was more precise in detecting nodules of 1–2 cm than CT scan [12]. However, 20–30 % of intrahepatic tumors, especially those smaller than 10 mm, are not diagnosed preoperatively with any technique. Preliminary data with positron emission tomography are not encouraging for detection of small nodules.
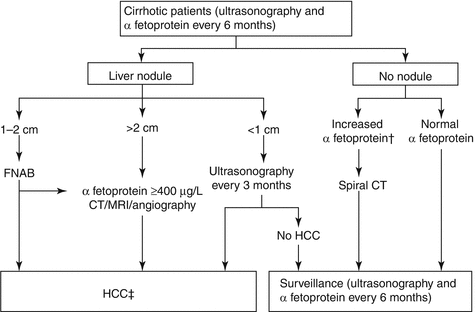

Fig. 1.2
EASL diagnostic algorithm
1.7 NCCN Guidelines
1.7.1 Imaging
Recommendations for imaging in the NCCN Guidelines, if clinical suspicion for HCC is high (e.g., following identification of a liver nodule on ultrasound or in the setting of a rising AFP level), are adapted from the updated guidelines developed by the AASLD [2]. The recommendations included in the NCCN Guidelines apply only to nodules identified in patients with liver cirrhosis. In patients without liver cirrhosis or known liver disease, biopsy should be strongly considered to confirm the diagnosis of HCC.
For patients with an incidental liver mass or nodule found on ultrasound, the guidelines recommend evaluation using one or more of the imaging modalities (at least a three-phase contrast-enhanced CT or MRI including the arterial and portal venous phase) to determine the perfusion characteristics, extent and the number of lesions, vascular anatomy, and extrahepatic disease. The number and type of imaging are dependent on the size of the liver mass or nodule.
Liver lesions <1 cm should be evaluated by at least a three-phase contrast-enhanced CT or MRI or contrast-enhanced ultrasound (CEUS) every 3–6 months, with enlarging lesions evaluated according to size. Patients with lesions stable in size should be followed with imaging every 3–6 months for 2 years (using the same imaging modality that was first used to identify the nodules) then returning to baseline surveillance schedule after 2 years of stability.
Liver nodules greater than 1 cm in size should first be evaluated with three-phase contrast-enhanced CT or MRI. Additional imaging is dependent on the pattern of classic enhancement observed. A finding of two classic enhancements is considered to be diagnostic of HCC, whereas a second imaging (the other CT or MRI) is recommended if there is only one or no classic enhancement pattern. If there are two classic enhancements following additional imaging, the diagnosis of HCC is confirmed. Additional confirmation through tissue sampling (core biopsy is preferred) is recommended if there is only one or no enhancement pattern for patients with liver nodules that are between 1 and 2 cm or greater than 2 cm. For patients with liver nodules between 1 and 2 cm, the NCCN Guidelines have included repeat three-phase imaging in 3 months as an alternative to core biopsy, if there is only one or no classic enhancement pattern following additional imaging.
1.7.2 Biopsy
A diagnosis of HCC can be noninvasive in that the biopsy confirmation may not be required. For example, liver nodules greater than 1 cm in size, the finding of two classic enhancement on either one of the recommended imaging modalities (three-phase contrast-enhanced CT or MRI) is sufficient to confirm the diagnosis of HCC. However, a core needle biopsy (preferred) or a fine-needle aspiration biopsy (FNAB) is recommended when zero or one classic arterial enhancement is observed by the recommended imaging method [18].
Nevertheless, the use of biopsy to diagnose HCC is limited by several factors including sampling error, particularly when lesions are less than 1 cm [2, 45]. Patients for whom a nondiagnostic biopsy result is obtained should be followed closely, and subsequent additional imaging and/or biopsy is recommended if a change in nodule size is observed. The guidelines emphasize that a growing mass with a negative biopsy does not rule out HCC. Continual monitoring with a multidisciplinary review including surgeons is recommended.
1.7.3 Serum Biomarkers
Although serum AFP has long been used as a marker for HCC, it is not a sensitive or specific diagnostic test for HCC. Serum AFP levels >400 ng/ml are observed only in small percentage of patients with HCC. In a series of 1158 patients with HCC, only 18 % of patients had values >400 ng/ml [46]. In patients with chronic liver disease, an elevated AFP could be more indicative of HCC in non-infected patients [47]. Furthermore, AFP can also be elevated in intrahepatic cholangiocarcinoma and some metastases from colorectal cancer [2]. AFP testing can be useful when combined with other test results to guide the management of patients for whom a diagnosis of HCC is suspected. An elevated AFP level in conjunction with imaging results showing the presence of a larger liver mass has been shown to have a high positive predictive value for HCC in two retrospective studies involving small number of patients [10, 48].
The updated AASLD guidelines no longer recommend AFP testing as part of diagnostic evaluation [4]. The panel considers an imaging finding of classic enhancement to be more definitive in this setting since the level of serum AFP may be elevated in those with certain nonmalignant conditions, as well as within normal limits in a substantial percentage of patients with HCC [49], which is in agreement with the updated AASLD guidelines recommendation. Four additional imaging studies (CT or MRI) are recommended for patients with a rising serum AFP level in the absence of a liver mass. If no liver mass is detected following measurement of an elevated AFP level, the patient should be followed with AFP testing and liver imaging every 3 months.
Other serum biomarkers being studied in this setting include des-gamma-carboxy prothrombin (DCP), also known as protein induced by vitamin K absence-II (PIVKA-II), and lens culinaris agglutinin-reactive AFP (AFP-L3), an isoform of AFP [15, 50–52]. Although AFP was found to be more sensitive than DCP or AFP-L3 in detecting early-stage and very early-stage HCC in a recent retrospective case-control study, none of these biomarkers were considered optimal in this setting [47]. A recent case-control study involving patients with hepatitis C enrolled in the large, randomized HALT-C trial who developed HCC showed that a combination of AFP and DCP is superior to either biomarker alone as a complimentary assay to screening [53].
References
2.
Bruix J, Sherman M. American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–2.PubMedCentralCrossRefPubMed
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree