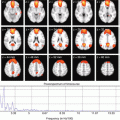
Fig. 2.1
Hypothetical model of dynamic biomarkers and clinical disease stage of AD. Modified from Sperling et al. [5]
Based on these advances in research findings, in 2011, the criteria of NINCDS-ADRDA have been updated by the National Institute on Aging/Alzheimer’s Association (NIA-AA) in the United States [3–6]. The aim of the new guidelines was to improve clinical diagnosis and establish research priority for the future. Then, in 2013, the American Psychiatric Association published DSM-5, in which dementia has been renamed “major neurocognitive disorder,” and a concept of “mild neurocognitive disorder” has been introduced [7]. The aim of this reclassification includes reducing stigma associated with dementia and providing the diagnostic guideline into line with current clinical practice.
2.2 The National Institute on Aging/Alzheimer’s Association (NIA-AA) Criteria
The NIA-AA diagnostic guidelines for AD were designed to reflect recent research which indicates that the pathophysiological process of AD takes place many years before cognitive decline or behavioral disturbance are noticeable [6]. The workgroup proposed three phases of AD progression over time—AD dementia, MCI due to AD, and preclinical AD. The core clinical criteria of the recommendations regarding AD dementia and MCI due to AD are intended to guide diagnosis in the clinical setting. However, the recommendations of preclinical AD are solely intended for research purpose and do not have any clinical implications at this time.
2.2.1 AD Dementia
The workgroup first proposed (1) criteria for all-cause dementia (Table 2.1) and then (2) criteria for AD dementia [4]. AD dementia involves memory, thinking, and behavioral symptoms that impair a person’s ability to function in daily life. The expert workgroup emphasized that memory impairment may not always be the most central characteristic of a diagnosis of AD and that biomarkers may be increasingly used to help improve diagnostic certainty, especially for research purposes.
Table 2.1
Criteria for all-cause dementia
1. Interference with the ability to function at work or at usual activities |
2. A decline from previous levels of functioning and performing |
3. Not explained by delirium or major psychiatric disorder |
4. Cognitive impairment is detected through a combination of history-taking and cognitive assessment |
5. The cognitive or behavioral impairment involves a minimum of two domains: |
a. Impaired ability to acquire and remember new information |
b. Impaired reasoning and handling of complex tasks and poor judgment |
c. Impaired visuospatial abilities |
d. Impaired language functions (speaking, reading, writing) |
e. Changes in personality, behavior, or comportment |
The following terminology for classifying individuals with dementia caused by AD was proposed: (1) probable AD dementia, (2) possible AD dementia, and (3) probable or possible AD dementia with evidence of the AD pathophysiological process. The first two were intended for use in all clinical settings. The third was intended for research purposes.
Probable AD dementia is diagnosed when the person meets all the core clinical criteria (Table 2.2). In persons who meet the core clinical criteria for probable AD dementia, documented cognitive decline increases the certainty that the condition represents an active, evolving pathologic process, but it does not specifically increase the certainty that the process is that of AD pathophysiology. Probable AD dementia with documented decline is defined as follows: evidence of progressive cognitive decline on subsequent evaluations based on information from informants and cognitive testing in the context of either formal neuropsychological evaluation or standardized mental status examinations. Probable AD dementia in a carrier of a causative AD genetic mutation is also defined as evidence of a causative genetic mutation (in APP, PSEN1, or PSEN2), which increases the certainty that the condition is caused by AD pathology.
Table 2.2
Probable AD dementia: core clinical criteria
1. Meets criteria for all-cause dementia |
2. Insidious onset: symptoms that have a gradual onset over month to years |
3. Clear-cut history of worsening of cognition by report or observation |
4. Initial cognitive deficits are evident and most prominent in one of the following categories: |
a. Amnestic presentation: impairment in learning and recall of recently learned information |
b. Nonamnestic presentations: |
• Language presentation: the most prominent deficits are in word-finding |
• Visuospatial presentation: the most prominent deficits are in spatial cognition |
• Executive: the most prominent deficits are impaired reasoning, judgment, and problem solving |
5. Diagnosis of AD should not be applied when there is evidence of another dementing illness |
Possible AD dementia is diagnosed when there is atypical course or etiologically mixed presentation. Atypical course has a sudden onset of cognitive impairment or demonstrates insufficient historical detail or objective cognitive documentation of progressive decline. Etiologically mixed presentation has evidence of (a) concomitant cerebrovascular disease, defined by a history of stroke temporally related to the onset or worsening of cognitive impairment, or the presence of multiple or extensive infarcts or severe white matter hyperintensity burden; (b) features of dementia with Lewy bodies other than the dementia itself; or (c) evidence for another neurological disease or a non-neurological medical comorbidity or medication use that could have a substantial effect on cognition.
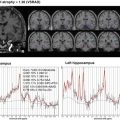
Probable or possible AD dementia with evidence of the AD pathophysiological process is diagnosed when there is biomarker evidence to increase the certainty that the dementia is due to AD (Table 2.3). The biomarkers are divided into two major categories: (1) the biomarkers of Aβ accumulation, which are abnormal tracer retention on amyloid PET imaging and low CSF Aβ42, and (2) the biomarkers of neuronal degeneration or injury, which are elevated CSF tau (both total and phosphorylated tau), decreased FDG uptake on PET in a specific topographic pattern involving temporoparietal cortex, and atrophy on structural MRI in a specific topographic pattern involving medial, basal, and lateral temporal lobe and medial and lateral parietal cortices [6]. Probable AD dementia with evidence of the AD pathophysiological process is diagnosed when the persons meet the core clinical criteria for probable AD dementia with biomarker evidence. If one of these biomarker categories is positive, the biomarker probability of AD etiology rises to intermediate, and both categories must be positive for the highest probability. The lowest probability is present if both categories are negative. Possible AD dementia with evidence of the AD pathophysiological process is the category for persons who meet clinical criteria for a non-AD dementia but who either have biomarker evidence of AD pathophysiological process or meet the neuropathological criteria for AD.
Table 2.3




AD dementia criteria incorporating biomarkers
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree