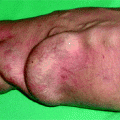
Fig. 10.1
Everybody (surgeon, nephrologist, radiologist) overlooked this small area of infection on contact with the scar of the anastomosis of this 6-week-old nonmaturing radial–cephalic fistula. Dilation of the juxta-anastomotic area resulted in severe bacteremia and 8 days in an intensive care unit
10.1.2.2 Relative Contraindications
Dilation is relatively contraindicated less than 1 month after access creation and across stenoses in the arterialized vein segment of fistulas (or prosthetic grafts) associated with distal ischemia or hyper flow.
Inflating a dilation balloon across the anastomosis of an access less than 1 month after surgery is likely to cause rupture of the unconsolidated anastomotic sutures resulting in a catastrophic leak that is generally extremely difficult to manage.
Dilating stenoses of the arterialized vein usually increases access flow and worsens the steal effect in cases with established distal ischemia.
Similarly flow further increases in an access with known hyper flow, magnifying the risk for cardiac insufficiency [1].
Some surgeons believe dilation should never be performed on stenoses in an access with cutaneous necrosis, and surgical revision should be the first and foremost treatment (Figs. 7.9 and 7.10). In contrast, our opinion is that surgical intervention before dilation can lead to perioperative unsalvageable access thrombosis and access loss given that cutaneous necrosis usually develops in areas of chronic venous hypertension and severe outflow stenosis. The danger of dilation before surgery is it can trigger venous and skin rupture leading to severe bleeding. Dilation-induced venous rupture can however be managed endovascularly by hemostatic suturing with or without covered stent placement. It is possible to reduce the risk of rupture by strictly adhering to certain rules of thumb during the pre-dilation phase: that is, avoid wetting or applying skin antiseptics over the necrotic patch to prevent it from being dislodged and making arrangements for the patient to be admitted and reviewed urgently by a surgeon.
Most importantly from a practical point of view, the biggest contraindication to dilation is a lack of necessary materials, for instance unavailability of stents to fix venous ruptures or dissecting occlusions. The same corollary applies when it comes to the lack of loop snares required for the retrieval of embolized wires or ruptured balloon fragments, which are extremely rare but still compelling complications.
10.1.3 Indications for Dilation
In general, once a significant stenosis (>50 %) has been identified and confirmed angiographically, the question always arises as to whether it should be dilated. Dilation is performed if the stenosis can explain the clinical symptoms or concerns prompting the referral for angiography or if it potentially threatens the short-term patency of the access.
In peripheral arterial diseases, once a decision is made for dilation, treatment should be as perfect and successful as possible, which implies frequent resort to stents to achieve that. Contrarily, some degree of residual stenosis is often accepted and desirable in dialysis fistulas. When a stenosis is treated for the first time ever, it is mandatory to bear in mind that a perfect dilation with no residual stenosis might result in a considerable increase in access flow, induce steal and reduce hand perfusion, or cause high-output cardiac insufficiency. In this regard, stenoses or chronic occlusions can be categorized into three groups: those that should be perfectly dilated, left untreated, or deliberately underdilated. The clinical context of each case and published outcomes from the literature play an important role in guiding the decision-making process.
Afferent arterial pre-anastomotic stenoses, which can be the cause of distal ischemic symptoms; venous anastomotic stenoses of prosthetics grafts; and symptomatic severe central vein stenoses or occlusions are the only stenoses that should be fully dilated. Dilation of other stenoses should always be preceded by the query as to whether full dilation is justified and if not by what quantum should they be underdilated and what should be the residual stenosis.
Stenoses that should never be dilated are essentially asymptomatic central vein stenoses (Fig. 10.2). Stenoses at the level of the subclavian, brachiocephalic veins, or superior vena cava (SVC) associated with significant debilitating and handicapping painful arm or facial edema are on the contrary strong indications for dilation.
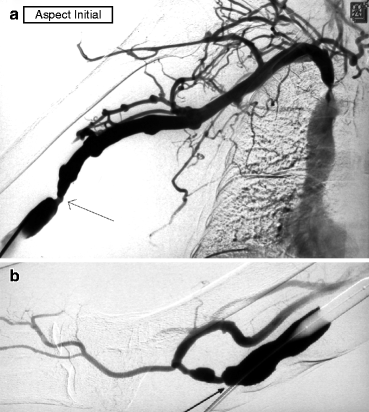

Fig. 10.2
(a) This right brachial–basilic transposed fistula is referred for venous hypertension in the needling segment, manifesting as prolonged bleeding after each dialysis session. Access flow is 550 mL/min. Angiography performed after an antegrade puncture of the juxta-anastomotic vein shows a stenosis at the terminal end of the superficialized arterialized vein segment (arrow). This is dilated. A right brachiocephalic vein stenosis is also demonstrated, but this is not treated in the absence of arm edema. (b) With the inflated balloon occluding venous outflow, the anastomosis is opacified by contrast reflux. Two nonsignificant juxta-anastomotic stenoses (arrow) are shown which are not dilated for three reasons: (i) the fistula is hyperpulsatile; (ii) these two stenoses are not the cause of the clinical symptoms; and (iii) they are probably protective, preventing further build up of intra-access pressure that might otherwise render the central vein stenosis symptomatic
Absence of edema despite severe stenoses or occlusion indicates that upstream collaterals, usually clinically very evident on the shoulder and upper chest, are well developed to ensure venous drainage into the right atrium. In the majority of cases, the accesses remain in good working order.
Dilation of asymptomatic central vein stenosis is most often an unnecessarily painful procedure unless done under deep sedation and can trigger a more rapid and severe restenosis accompanied by arm edema, particularly when the first intervention involved inappropriate use of stent which obliterated the collaterals. The strategy of not dilating asymptomatic cases is strongly supported by two separate series by Levit and Renaud [2, 3]. Raised intra-access venous pressure, mild to moderate non-debilitating arm edema, and collateral circulation near the shoulder and upper chest area are clinical clues to central vein stenoses but not indications for dilation. Exceptionally, dilation is strongly considered when severe venous hypertension results in difficult hemostasis postdialysis after needle removal.
Central vein stenoses, whether symptomatic or asymptomatic, should be mandatorily dilated as well if angiography shows retrograde flow into the ipsilateral internal jugular vein in the direction of the cerebral venous sinus and drainage into the contralateral internal jugular vein. Intracranial hypertension and pseudotumor cerebri-like symptoms are likely to develop if the stenosis is not treated to decompress intracranial collateral blood flow [4].
Peri-anastomotic stenoses (Fig. 10.2b) found in accesses referred primarily for venous hypertension, hyper flow, or outflow stenoses (e.g., cephalic arch stenoses or stenoses in the superficialized segment of basilic vein transposition) should also frequently be left undilated. These inflow stenoses are protective and beneficial as they keep in check excessive rise in venous pressure and access flow, thereby preventing distal ischemia or cardiac insufficiency. They should only be dilated when they appear less than 2 mm in diameter or they are severe enough to give rise to a flat or poorly thrilling AVF after opening up of outflow lesions. The ideal is to assess the hemodynamic significance of the inflow stenosis by performing a per-procedural blood flow measurement using a Transonic catheter or duplex ultrasound after the outflow stenosis has been dilated [5].
There is a group of stenoses that should be deliberately underdilated, and they comprise all peri-anastomotic stenoses in all upper arm accesses and in radial–cephalic fistulas with concurrent ulnar artery lesions. A 5- to 6-mm balloon is used to ensure underdilation and limit the risk of distal ischemia or raised access flow that can in return increase retrograde distal arterial steal. The size of the dilation balloon is usually increased by 1 mm during each redilation if no distal ischemia intervenes between dilations.
Other stenoses in upper arm accesses should also be underdilated on first intervention in all patients at risk of distal ischemia (i.e., diabetics, smokers, and elderly), particularly if there are demonstrable distal arterial anomalies on angiography. The size of the dilation balloon used for dilation of cephalic arch stenoses or stenoses in the outflow of transposed basilic veins is thus best limited to 7 mm. This can then be gradually increased by 1 mm after each restenosis as tolerated after fully ensuring that no sign of transient distal ischemia occurred after the precedent dilation. It is risky to fully dilate or place a stent across such stenoses during the first intervention.
Undersizing the dilation balloon is also recommended for stenoses found in dialysis cannulation areas once there exists an abnormally very thin skin cover over the arterialized vein. Full dilation in this instance can cause both vein and skin rupture, resembling a thin sharp knife incision (Fig. 10.3). Such rare complications can be managed initially endovascularly by balloon tamponade, cutaneous suturing, and covered stent placement followed by semi-elective revision surgery [6]. Our own center has seen three such cases in the last 5 years.
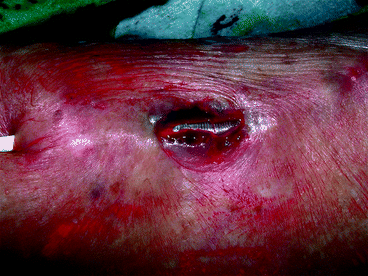

Fig. 10.3
Both vein and skin rupture, resembling a thin sharp knife incision, resulted from dilation to 7 mm of a stenosis in the weakened cannulation area of this superficialized radial–cephalic fistula. The inflated Conquest balloon was visible through the breach
It is a prudent strategy to deliberately underdilate stenoses, wherever they are located, in patients having concurrent ipsilateral severe but asymptomatic central vein stenoses. A rise in access flow after dilation under such scenarios can overwhelm the collateral network ability to maintain venous drainage and precipitate painful arm swelling and hence turn the asymptomatic into a symptomatic stenosis (Fig. 10.4a–d). The central vein stenoses will then have to be dilated, knowing well the risk of dilation-induced early restenosis.
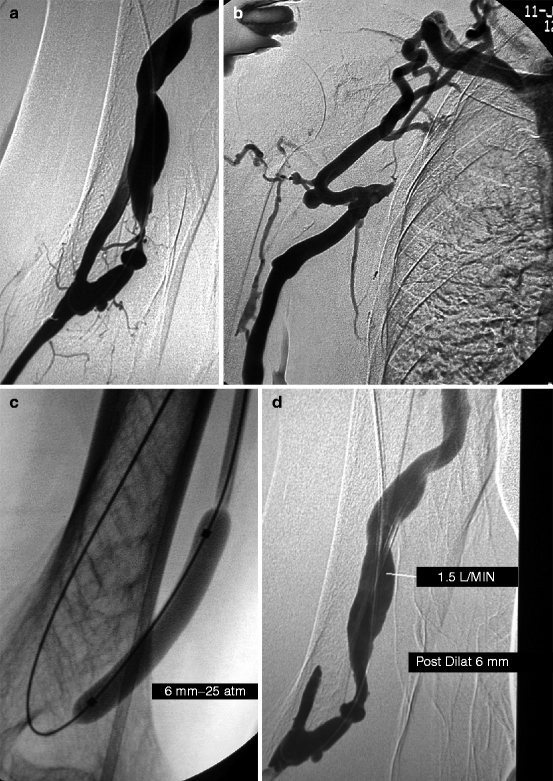

Fig. 10.4
(a) This transposed brachial–basilic fistula was referred for needling difficulties. Access flow was 500 mL/min by Transonic®. Angiography was performed after retrograde puncture of the fistula and advance of a 4-F catheter across the anastomosis into the brachial artery. Of note was a severe juxta-anastomotic stenosis which correlated with the clinical signs and threatened access patency. (b) Angiography also showed a long subclavian vein occlusion. The absence of arm edema explained by the rich collaterals made recanalization and dilation of this chronic occlusion unnecessary. However, full dilation of the juxta-anastomotic stenosis might increase access flow too much and overwhelm the ability of the collaterals to compensate venous outflow and hence trigger arm edema. (c) The juxta-anastomotic stenosis was therefore deliberately underdilated with a 6-mm balloon only. (d) Post-dilation angiography showed significant residual stenosis. The need for or against further dilation with a larger balloon was ascertained by measuring intra-access blood flow using a Krivitski thermodilution catheter [5]. In this case, access flow was 1.5 L/min, and the inflow stenosis was thus not dilated with a larger balloon. The patient represented a few weeks later with mild arm edema, but the symptoms were not handicapping enough to warrant subclavian vein recanalization. The edema stabilized, and the patient passed away 23 months later from an unrelated cause
10.1.4 Basic Techniques of Angioplasty
10.1.4.1 Background
It is very important to be clear and methodical about the route and safety precautions to adopt once a significant stenosis is identified on angiogram and indication for dilation rationalized. On rare occasions, dilation can result in complications like severe vessel rupture. A cautious approach ensures that the right tools, measures, and skills are available to handle serious complications when they do happen. This entails the interventionist being able to easily deploy an occluding balloon in an afferent artery to arrest flow into a ruptured vessel segment and leaving enough length between the introducer-sheath tip and stenosis to allow placement of a stent. The arterialized vein of an AVF is preferably accessed in a segment that can be most easily punctured by palpation and as far away from the stenosis of interest as possible. Dilation of peri-anastomotic stenoses involves placing a guidewire in the proximal artery, which is not always an easy maneuver in forearm AVFs.
10.1.4.2 Cannulation
The vessel cannulated for diagnostic angiography may not always be the best route for deploying the materials required for dilation particularly when this happens to be the brachial artery. In the case where the vein or graft is punctured, it is necessary to subcutaneously tunnel the needle 1–2 cm away from the wall of the vessel before entering it. This avoids direct access to venipuncture that can potentially lead to secondary pseudoaneurysm formation. It also makes hemostasis of the puncture site by compression at the end of the procedure easy without risking access lumen occlusion. Flat or low flow and therefore not easily palpable AVFs are cannulated after placing a tourniquet at the mid-upper arm or under echographic guidance.
An 18-G cannula and 0.035-in. angled-tip or “J” hydrophilic guidewire make up the primary tools required to cannulate the majority of accesses and allow placement of an introducer-sheath (usually 5–8 F depending on the size of dilating balloon to be used).
10.1.4.3 Passing Stenoses
Angled-tip hydrophilic guidewire can be steered across most stenoses but should be avoided in heterogeneous segmental stenoses. Its passage through long occlusions and stenoses adjacent to aneurysmal dilatations is also largely challenging if not impossible.
Heterogeneous stenoses, both long and short, are encountered only in arterialized veins which have never undergone dilation. They are due to pre-dialysis venipuncture-induced endoluminal trauma, transient thrombosis, and partial spontaneous recanalization (Fig. 10.5a–d). A hydrophilic guidewire in this situation is more likely to false track, create a false lumen, and cause irreversible vessel wall dissection. Dissections antegrade to blood flow are usually more sinister. A more steerable and atraumatic guidewire, in the like of the 0.014-in. prototypes used in renal artery stenosis or coronary angioplasties (Spartacore, BMW, Whisper, etc.), supported by a 5-F vertebral diagnostic catheter stands a greater chance of passing through such stenoses.
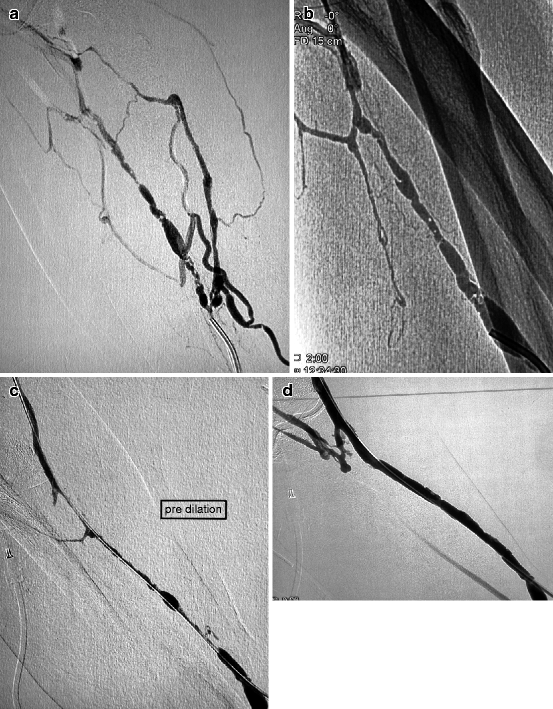

Fig. 10.5
(a, b) This 5-week-old left radial–cephalic fistula presented with nonmaturation caused by a segmental heterogeneous stenosis in the upper third of the forearm. The vein was punctured antegradely near the anastomosis after application of a tourniquet in the upper arm. The brachial artery was not punctured as the patient was on oral anticoagulants. (c) A combination of a 5-F vertebral catheter and 0.014-in. guidewire allowed a meticulously slow crossing of the stenosis. Use of a hydrophilic guidewire in this long heterogeneous stenosis would have resulted in false tracking and venous dissection. (d) Excellent result was obtained with 6-mm balloon dilation, and the fistula was needled a week later. Redilation was performed 4 months later with a bigger balloon
The technique is to place the vertebral catheter mounted on a hydrophilic guidewire in contact with the stenosis and then to maneuver through it the 0.014-in. guidewire several times until its tip is in the axis of stenosis and traverses it (Figs. 10.6a–c and 10.7a–e). The 0.014-in. guidewire has a very soft and flexible tip which can penetrate stenotic segment mm by mm before hitting against a new area of resistance without causing trauma. The 5-F vertebral catheter should follow the trajectory of the guidewire tip and be maneuvered by subtle changes of angulation so that the guidewire can be gently steered through the entire stenosis at alternating angles along the axis of the access lumen. Some interventionists prefer railroading a 3-F microcatheter across the 5-F catheter to provide better support for the guidewire.

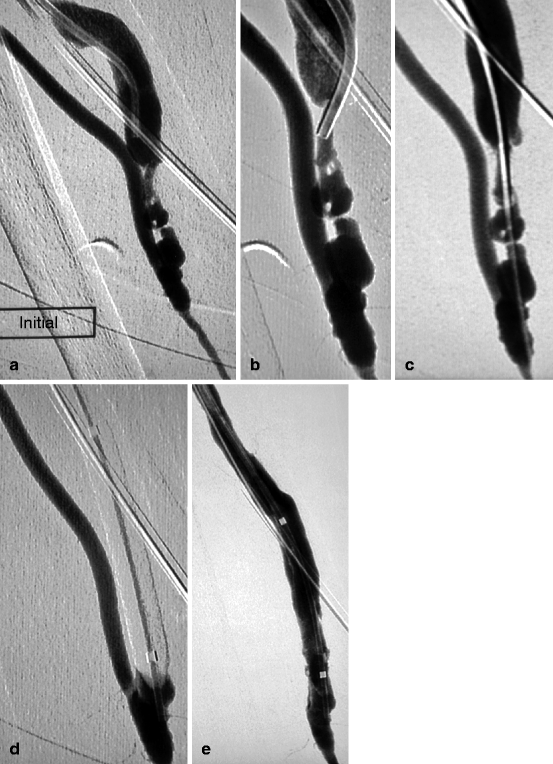

Fig. 10.6
(a) This 6-week-old right radial–cephalic fistula presented with nonmaturation due to a long severe stenosis of the arterialized vein. It was punctured retrogradely at the elbow with the help of a tourniquet. A 4-F mammary catheter was advanced over a guidewire to come in contact with the stenosis. All attempts at crossing this valvular hypertrophy (arrow) along the luminal axis failed. (b, c) The stenosis was hence traversed after a retrograde distal radial artery puncture and use of a 4-F vertebral catheter and 0.014-in. guidewire combination

Fig. 10.7
(a) This left radial–cephalic fistula presented with nonmaturation caused by a tight heterogeneous juxta-anastomotic stenosis. (b) A 5-F vertebral catheter was perched at the apex of the stenosis. (c) A 0.014-in. guidewire supported by the vertebral catheter was steered through the challenging stenosis and the 5-F catheter then pushed over it. (d) The 0.014-in. guidewire was replaced by a 0.035-in. guidewire over which was mounted a 6-mm balloon. The guidewire was removed after inflation of the balloon, and injection through the lumen of the balloon checked that dilation did not induce any stenosis (by pushing of debris) or spasm in the proximal artery. (e) Completion angiography was made through the balloon lumen
Passage of the guidewire through the stenosis can be facilitated by a simple technique of stretching the overlying skin and vein between two fingers in order to modify its morphology and angulation of the stenosis. Once the guidewire has completely crossed the stenosis, the catheter is advanced beyond the stenosis, and the initial 0.014-in. guidewire is replaced by an angled-tip 0.035-in. metallic “safety” guidewire which is sturdier, more secure, and therefore unlikely to slip out. Failed attempts at crossing the stenosis with a 0.014-in. guidewire require a change of strategy which should include attacking the stenosis from a different direction or location (femoral vein in the case of central vein stenosis or brachial or distal radial artery). As a last resort, a 0.035-in. straight-tip hydrophilic guidewire can be used to attack the stenosis, preferably in a direction retrograde to blood flow. A vessel wall dissection in the opposite direction to blood flow is less likely to result in loss of the access by thrombosis and may thus leave the option open for surgical intervention.
The maneuvering of an angled-tip hydrophilic guidewire through a stenosis adjacent to an aneurysm or pseudoaneurysm can be challenging. The guidewire tends to hit a dead end and coils up inside the aneurysmal formation. A straight-tip guidewire particularly has an advantage and is preferred as it can be more easily maneuvered into the neck of the aneurysm and aligned in the axis of the stenosis. Compression and stretching of the aneurysm are also useful techniques that often help get the guidewire across the stenosis (Fig. 10.8a, b). A rather exceptional alternative but workable technique is to advance the vertebral catheter without a leading guidewire across stenosis found between two aneurysms. These stenoses are usually soft and can accommodate the tip of a 5-F catheter. The guidewire should follow once the catheter is in the lumen of the stenosis. It must be cautioned that this is perhaps the only instance when the golden rule that a catheter should never be advanced into any lesion without a leading guidewire is breached.
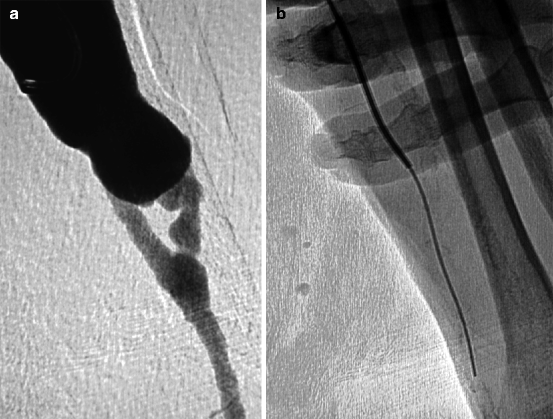

Fig. 10.8
(a) Low flow in this left radial–cephalic fistula of many years can be explained by a juxta-anastomotic stenosis. After retrograde puncture of the vein, it was impossible to cross the stenosis as the guidewire kept coiling up in the aneurysmal vein. (b) The trick was to advance a straight-tip hydrophilic guidewire supported by a 5-F vertebral catheter while compressing and stretching the aneurysm with two fingers to prevent guidewire from coiling. The arterial calcification was used as a landmark to ascertain whether the guidewire crossed the anastomosis
An undulating or tortuous arterialized vein segment can be traversed by an angled-tip hydrophilic guidewire with a bit of effort. The guidewire initially assumes the curvature of the vein as it is advanced but usually straightens the vessel undulations once it reaches the straight normal segment further downstream.
Segmental occlusions should be attacked with a vertebral catheter/straight-tip hydrophilic 0.035-in. guidewire pair, preferably against the direction of blood flow whenever this is possible. The trick is to prod at the occlusion and find the true lumen either upstream or downstream depending on the direction of attack in relation to blood flow. The risk of failure and creating a false guidewire track in the process is in the order of 20 % in chronic occlusions, whereas technical failure in passing stenoses is rare in well-experienced hands.
Once the guidewire has crossed the stenosis, its tip should be placed either in the brachiocephalic trunk, superior vena cava, contralateral internal jugular vein, inferior vena cava, or hepatic vein. The guidewire tip should never be left in the right atrium or ventricle during interventions on outflow vein stenoses where it is likely to induce arrhythmias or cause potentially fatal endocardial and myocardial trauma during forceful manipulations and torquing. A number of such complications have previously been reported.
All these tips and tricks on maneuvering around stenoses should however not be a substitute for steadfast and adequate hands-on training on real-life cases and supervision by well-experienced seniors.
10.1.4.4 Balloon Dilation
The next phase after steering a guidewire through a stenosis is balloon dilation. Dilation is potentially a very painful procedure, and it is essential to administer adequate analgesia as patients exhibit wide variations in pain threshold. Whenever possible, the stenosis and its surrounding area should be infiltrated subcutaneously with 1–2 % lidocaine. Administration of local anesthetics is not technically possible during dilation of deeply seated stenoses found on the medial aspect of the upper arm, cephalic arch, axillary, and upper chest areas.
The next consideration is the choice and sizing of the dilation balloon (diameter and length). If the aim of dilation is a perfect result, then no residual stenosis should remain such that from the completion angiogram it is difficult to tell where along the access the initial stenosis was located (Fig. 10.9a–c). In real practice, complete absence of residual stenosis is more ideal as some stenoses tend to reform immediately once the balloon is removed, a phenomenon known as elastic recoil. There exist three basic rules when it comes to selecting the appropriate dilation balloon size: the balloon diameter should be 1–2 mm larger than that of the reference vessel adjacent to the stenosis, high pressure balloons need to be readily available to replace conventional balloons when results are not satisfactory with the latter, and covered stents of different sizes should be kept in stock in anticipation of any major vessel rupture.
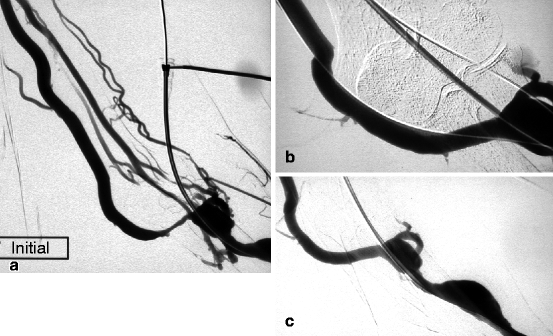

Fig. 10.9
(a) This left radial–cephalic fistula presented high venous pressure in the forearm as a result of a stenosis in its basilic vein outflow (median cubital vein). The cephalic vein outflow was occluded, and the perforating vein network was not well developed. (b) Good result was obtained after 9-mm balloon dilation (guidewire still in situ). (c) The perfect result was confirmed after guidewire removal. From the completion angiogram, it is impossible to tell where the initial stenosis was
Dilation of venous anastomotic stenoses in prosthetic grafts (Goretex®, Flixene®, Propaten®, etc…) is perhaps the simplest form of dilation and shall be used as an example for discussion below. Prosthetic grafts are generally 6 mm in diameter. Venous anastomotic stenoses are usually dilated at a minimum with a 7 mm or more commonly with 8 mm dilation balloons. Rarely, a 10-mm balloon may be considered when the downstream vein segment is large enough to accommodate it. The standard balloon length is 4 cm. Shorter versions tend to slip out of the stenosis grip during inflation. The correct positioning of the balloon with respect to the stenosis needs to be verified on fluoroscopy, and the right placement of the guidewire in the lumen of the main vein rather than a smaller tributary or vessel wall should be verified by angiography. The balloon is connected to a manual inflation device or manometer which is filled with iodinated contrast diluted to a minimum 75 % by saline. The manometer has a needle gauge which indicates the pressure generated in the balloon lumen on inflation. Dilation is always performed under fluoroscopic guidance until there is complete effacement (elimination) of the balloon waist, indicating complete dilation. Balloon inflation transmits an incremental radial force around the stenosis tearing away the fibrotic tissue and potentially causing profound pain. The balloon rated burst pressure (RBP), which is the inflation pressure beyond which the balloon bursts in vitro, must be considered beforehand so that change to a higher pressure balloon can be made when full effacement is not obtained at the RBP of conventional balloon.
Up to 2012, there are on the international market three types of the so-called ultrahigh pressure (UHP) balloons that can take in inflation pressures exceeding 25–30 atm although their official RBP is slightly lower: Mustang® (Boston Scientific) and Dorado® (Bard) can usually be inflated to 25 atm and the Conquest® (Bard) to 30 atm. Blue Max® (Boston Scientific) and Powerflex Extreme® (Cordis) are relatively older UHP prototypes and used to be favored in a not too distant past being the first balloons that broke the 20-atm UHP defining barrier. Blue Max® is still used in the dilation of anastomotic and juxta-anastomotic stenoses in forearm AVFs. It has a shorter and more compliant tip-to-end-of-balloon length (balloon shoulder) than Mustang or Conquest and hence ideal for anchorage in the first centimeter of the venous post-anastomotic segment of radial–cephalic AVFs.
Globally, based on published literature, about 25 % of AVF stenoses require UHP balloons for full effacement [7]. Whether an ultrahigh pressure balloon is used for primary dilation of stenoses or only after failure of conventional balloons (RBP less than 15 atm) is at the interventionist’s discretion. First-line use of UHP balloons saves time but does not necessarily provide better outcome when compared to conventional balloons [8]. They are more expensive, less compliant, harder to deflate, and require sometimes larger introducer-sheaths.
The usual ultimate aim of dilation is 100 % immediate success as denoted by full effacement of the waisting on the balloon. According to a randomized control trial, good angiographic result is more achievable with prolonged balloon inflation (3 min) than standard immediate balloon deflation [9]. However, beyond immediate cosmetic results, prolonged balloon inflation does not confer any overall long-term survival advantage to dialysis accesses. Standard inflation and immediate deflation are on the contrary very suited for a heparin-free protocol as prolonged balloon inflation without heparinization (usually 3,000 units of heparin in one bolus) is associated with a higher rate of thrombus formation though this can be mitigated by frequent saline flushing (1–2 mL every minute) of the outflow vein.
It is sound practice to compress the arteriovenous anastomosis during balloon deflation to prevent the sudden transmission of high pressure flow around the weakened dilated zone and hence minimize the risk of venous rupture. The skin overlying the dilated segment should be examined for swelling or hematoma formation, suggestive of venous rupture, which is usually accompanied by painful sensation.
10.1.4.5 Immediate Post-dilation Angiography
A post-dilation angiogram is obtained after balloon withdrawal with guidewire kept in situ. In the case of forearm AVFs with peri-anastomotic stenoses, contrast is injected either through the brachial artery if it was punctured for diagnostic angiography or through a catheter advanced over the guidewire into the proximal radial artery.
Four possible scenarios may play out post dilation:
1.
A perfect result with no residual stenosis, which should be confirmed by a completion angiography after removal of the guidewire (Fig. 10.9a–c). The procedure is then considered complete. The guidewire sometimes behaves like a stent and may wrongly give the impression of a superb successful dilation when left in situ during completion angiography although it turns into a major recoil or occlusion as soon as the guidewire is removed (Fig. 10.10a–h). Elastic recoil is such that one may observe satisfactory dilation in one stenotic segment but which may yet disappear 10 min later on completion angiography performed after dilation of other stenoses.
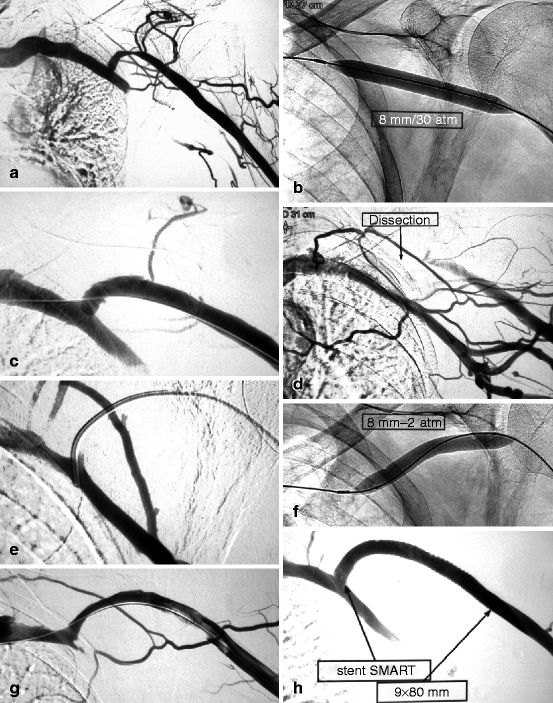

Fig. 10.10
(a) This cephalic arch stenosis was the cause of high venous pressure in this left brachial–cephalic fistula with an access flow of 530 mL/min. (b) The stenosis was dilated with an 8-mm Conquest® balloon. (c) The post-dilation image looked satisfactory though there was a slight dissection in the convexity of the cephalic arch. (d) On removal of the guidewire, there was sudden occlusion of the cephalic arch which could only be explained by a worsening dissection. (e) Without much difficulty, a guidewire was steered across the correct venous lumen. The successful insertion of the diagnostic catheter into the subclavian vein further confirmed that the guidewire was indeed in the venous lumen, not in a false track. (f) Prolonged balloon tamponade at 2 atm for 2 min using an 8-mm balloon was performed. (g) Angiography after a further 2-min dilation showed mild residual stenosis and small upstream thrombus. (h) The residual stenosis and thrombus were treated by placement of a long 9-mm diameter Smart® stent
2.
A residual stenosis of less than 30 % is still considered acceptable and a technical success, especially if it is the access first ever (primary) dilation. The stenosis can be redilated with the same balloon for a longer duration of 2–3 min (remembering to administer heparin or flush the introducer port with saline every minute). Upsizing the dilation balloon diameter by 1 mm may be considered in the absence of vessel damage or transient extravasation caused by the precedent smaller balloon. Stent placement is strongly contraindicated whenever residual stenosis is less than 30 % after a primary dilation. Dilated stenoses with less than 30 % residual stenosis may remain largely stable for months, while it is not uncommon to see more perfect results restenosing within 3 months.
3.
A residual stenosis of more than 30 % is considered a technical failure and automatically warrants the use of a larger balloon (upsized by 1–2 mm) (Fig. 10.11a–d). A stent may be placed across the stenosis if there is persistent lack of favorable response to balloons of larger size as long as there are no local anatomical contraindications to it. The immediate good result conveyed by stents is unfortunately rarely long lasting.
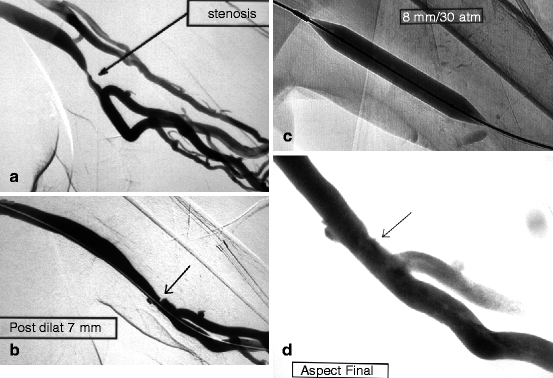

Fig. 10.11
(a) This left brachial–axillary prosthetic graft, as expected, developed a venous anastomotic stenosis triggering reflux in the deep brachial veins. (b) There was some residual stenosis (arrow) after 7-mm balloon dilation. (c) Dilation was repeated with a larger (8-mm) Conquest® balloon at an inflation pressure of 30 atm. (d) The result after 8-mm dilation (arrow) was satisfactory but not perfect. Reflux into the deep brachial veins had ceased
4.
Contrast extravasation on angiography suggests vascular rupture which clinically can also manifest as a localized tender hematoma. Vascular rupture is relatively easily managed by balloon tamponade whereby the dilation balloon is reinflated to 2 atm across the rent in the vessel for 5 min (Fig. 10.12). The hematoma is compressed manually at the skin, while attempts are made to reverse the effect of anticoagulation with dose-adjusted protamine sulfate if heparin has been administered. The outflow vein must be flushed with saline every minute (through the lumen of the inflated balloon) to avoid thrombus formation. It is also important to verify, through control angiography, after injecting contrast in the side port of the introducer-sheath, that the balloon is straddling the vessel defect well and that there is no extravasation upstream to the balloon. Low-pressure balloon tamponade of 5 min duration can be tried three times, failing which a covered stent should be placed to seal the defect [10, 11]. Bleeding during stent deployment is more effectively controlled by placing a small occlusive balloon in the feeding artery than getting an assistant to manually occlude the anastomosis, especially if the anatomy of the access limits the proper application of the latter technique (Fig. 10.13a–e). Usually, a retrograde cannulation of the brachial artery offers the easiest and fastest way to placing the occlusive balloon into the afferent artery. Alternatively, a blood pressure cuff inflated at suprasytolic pressure at the upper two-thirds of the upper arm can serve as a hemostatic tourniquet. However, blood pressure cuffs are not always effective and can cause pain and discomfort across the arm. The patient should never leave the intervention suite with an ongoing leak, no matter how mild, still visible on angiography despite all measures to stop it. In the rare occasion where everything else fails, a second stent can be considered.
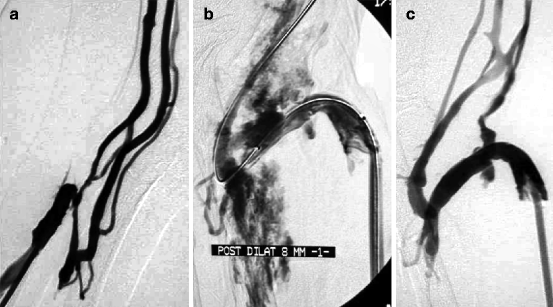
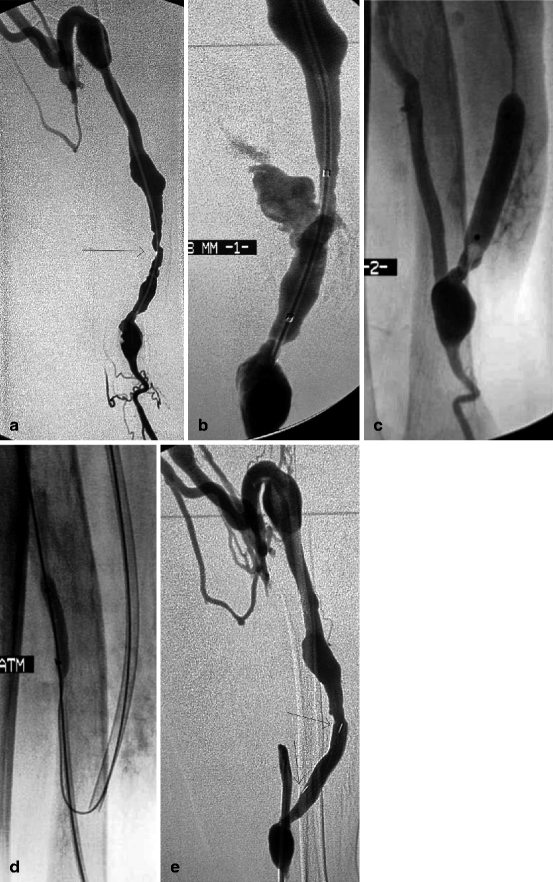

Fig. 10.12
(a) Venous hypertension in this right radial–cephalic fistula was explained by a stenosis of the perforating vein at the elbow. No venous basilic or cephalic stump was visible, and the only solution was to dilate this perforating vein despite the unfavorable anatomy. (b) Dilation to 7 mm resulted in a clear venous rupture which was by chance sealed by balloon tamponade. (c) Effective placement of a stent in this location might have been impossible

Fig. 10.13
(a) Low flow in this 3-year-old left radial-cephalic fistula was caused by two stenoses (arrow) near the anastomosis. The two cannulation sites were noticeable by their slight focal aneurysmal degeneration. The venous outflow was solely through a well-developed perforating vein. (b) Balloon dilation at 8 mm (probably overdilation) resulted in dramatic venous rupture which was not controlled despite three attempts at balloon tamponade over intervals of 10 min. The only solution left was to place a stent. The question was how to interrupt blood flow through the rent while at the same time placing the stent given no “safety” guidewire had been placed in the proximal artery at the beginning. (c) The anastomosis and afferent artery were opacified by reflux of contrast through the lumen of the hemostatic balloon. The anastomotic chamber was seen to be large enough and therefore accessible. (d) The anastomosis was thus directly punctured and the proximal radial artery catheterized before placing and inflating a 4-mm balloon to occlude arterial inflow. The 8-mm balloon was then deflated and removed before a self-expanding covered stent (Passager®) was deployed across the venous rent. (e) Extravasation ceased after covered stent placement (arrows). No further risks were taken, and, therefore, the residual stenosis near the anastomosis was not further dilated with a bigger balloon. No further intervention was required on this fistula until the patient’s demise 26 months later
Vascular rupture at arteriovenous anastomosis is the rarest but probably the most disastrous complication of balloon dilation. The narrow anastomotic chamber can accommodate for balloon tamponade but does not favor effective covered stent placement when the former measure fails. Access loss at this stage is inevitable. For the greater majority of such cases, bleeding control rather than access salvage should be the main aim of therapy. Hemostasis can be achieved by coil embolizing both the proximal and distal arteries of radial–cephalic AVFs. In the case of accesses fed by the brachial artery, the balloon across the ruptured anastomosis should be reinflated and the patient transferred urgently to theater for surgical repair of the artery.
In the cases where there is sufficient length of post-anastomotic brachial artery stump, this can be cannulated retrogradely and a covered stent deployed in the brachial artery to straddle the ruptured anastomotic segment.
Dilation-associated vascular rupture can occur at any anatomical location within the access, but is more frequently seen in the radial artery, juxta-anastomotic and transposed segments of veins, and at the cephalic arch [12]. It has never been described at the level of the subclavian or brachiocephalic veins.
Guidewires may induce venous spasm, just like a dilation balloon inflated across a point of vein bifurcation can do the same, even on the uncatheterized venous branch. It is not always easy to differentiate spasm from occlusive wall dissection though the former always responds to low-pressure (2 atm) balloon dilation or propagates further downstream or upstream into the proximal artery. The introducer-sheath is known to cause spasm at its point of entry into the vein. Such spasms are more frequently seen in newly created AVFs, and they can become occlusive. They sometimes develop on a relatively long venous segment and may cause pain. They may be eventually the only explanation to recurrent difficulties in cannulation for dialysis.
The AVF in this case may need to be cannulated from a different location to allow the introduction of a balloon to recanalize the occlusion and reestablish flow since local injection of vasodilators like nitrates is usually ineffective. Older AVFs, usually more than 1 year, rarely develop such spasms as their walls are thicker and more rigid from arterialization.
10.1.4.6 End of Dilation Procedure
As a routine, the completion angiogram should be read with meticulous attention from anastomosis to central veins before removal of the introducer-sheath to ensure no subtle anomaly that may compromise the function of the access is missed and left unrectified. The presence on clinical examination of the access of a faint thrill or abnormal hyperpulsatility should raise the suspicion of an anomaly and prompt even more detailed review of the completion angiogram for corroborative lesions. The interventionists should put themselves in the place of dialysis nurses who will have to cannulate the access the same or following day.
Once the introducer-sheath is removed, the cannulation tunnel, but not the wall of the access, is compressed to stop bleeding. Manual compression rarely lasts longer than 10 min in cases where introducer-sheaths smaller than 8 F are used. Punctures made by larger introducer-sheaths, in patients on anticoagulants and during all thromboaspiration procedures, bleed longer and should be closed using a U-shaped suture technique subtending a cut end of the introducer-sheath dilator [13] (Fig. 12.6a–f). Any swelling that forms immediately around the dilated segment should be carefully examined to make sure it is due to a transient hematoma arising from the dilation and not an ongoing vascular leak causing the skin to harden.
A few patients do complain of diffuse or localized pain once all materials have been removed. In the majority of cases, the pain is not related to any rupture and can be managed with topical, oral analgesia, or even local anesthetics. Such cases should be discussed as to the course of analgesia and monitoring and passed on to the attending nephrologists.
10.1.5 Technical Details
10.1.5.1 Anticoagulants and Antiplatelets
The use of heparin in routine dilation is usually not necessary to prevent thrombosis after interruption of access flow by the inflated balloon, but it remains interventionist and lesion dependent. All it takes in some patients is 30 s of balloon occlusion for thrombi to form downstream. These thrombi are very fresh and can thus be easily aspirated through a 6-F catheter or if small in size can be discretely pushed into the pulmonary circulation. The simplest and safest way to prevent access thrombosis during dilation is to flush boluses of saline (in the order of a few milliliters) at 1-min intervals through the introducer-sheath side port. It is even more imperative to flush the immediate outflow vein when the balloon is left inflated for a few minutes like during balloon tamponade. Some interventionists more concerned about access thrombosis independent of the complexity of the case prefer administering 2,000–3,000 IU of heparin particularly when the patients are not on any oral anticoagulant. Great caution should be exercised with the adjunct use of heparin during transbrachial artery puncture or procedure.
The use of oral anticoagulants or antiplatelets post-dilation for better outcomes to date is not supported by any clinical study. Randomized control trials however have shown that there is a role for antiplatelets like clopidogrel and dipyridamole in maintaining early primary patency of newly created AVFs and prosthetic grafts [14, 15].
10.1.5.2 Hypertrophic Valves Associated Stenoses
These venous stenoses are characterized by their central position and slit-like opacification surrounded by the concave lumps on each side which are the hypertrophic valves, in contrast to the full opacification seen beyond the valves (Fig. 10.14). Passing a guidewire across such stenosis is as challenging as getting it through stenoses associated with aneurysms. The trick is to torque the stiff end of a reversed hydrophilic guidewire through the slit. There is always a small risk of subintimal dissection and false guidewire tracking.


Fig. 10.14
This is a typical image of a stenosis due to a hypertrophic valve in a forearm cephalic vein
10.1.5.3 Retrograde Cannulation at the Elbow
Retrograde cannulation of either the cephalic or basilic vein in the lower third of the upper arm is usually performed to access venous stenoses located in the forearm using the catheter–guidewire pair. It often does produce some surprises. The venous anatomy at elbow consists of both superficial and deep veins connected by a perforating vein. The guidewire may be wrongly steered into a deep vein, from where it becomes impossible to find the way to the stenosis of interest in the superficial vein. The trick is to opacify the stump of the cephalic vein that leads to the stenosis by performing local test angiographies with a tourniquet applied near the shoulder. The cephalic vein segment that needs to be catheterized may sometimes be located more laterally or higher than the interepicondylar line. Test angiographies from the brachial artery (in the case where it has already been retrogradely punctured) may help give a better definition of elbow venous anatomy and guide the direction to take. A combination of 5-F vertebral diagnostic catheter and hydrophilic guidewire guarantees the best chance of reaching and crossing the stenosis.
10.1.5.4 Multiple Stenoses
The general rule for dilating accesses with multiple significant stenoses is to start with the most central or downstream (outflow) stenosis and end with the stenosis closest to the anastomosis (inflow). Arterial stenoses should be dilated after venous stenoses. The reason is that any dilation can result in venous rupture that is usually controlled successfully by prolonged balloon inflation. If an upstream stenosis is then dilated (i.e., a stenosis located between the arteriovenous anastomosis and the initially dilated venous stenosis), inflating the balloon stops flow considerably, decreases downstream pressure and helps seal the leak. On the contrary, while dilation of a downstream stenosis (i.e., a stenosis located between the initially dilated venous stenosis and central veins) also stops fistula flow, it nonetheless considerably increases pressure in the upstream segment and facilitates reopening of the previous breach.
10.1.5.5 High Pressure Balloons
At least 50 % of venous stenoses fully dilate at a balloon inflation pressure of 15 atm according to one published report, which implies that any type of balloon, even the most basic, can be used for primary dilation of venous stenoses [7]. The same study also showed that 20 % and 10 % of stenoses in AVFs and prosthetic grafts, respectively, require a balloon pressure above 20 atm to fully dilate. It is therefore indispensable to also have in the intervention armamentarium ultrahigh pressure balloons. The Conquest® balloon (Bard) can withstand inflation pressure greater than 30 atm, but higher pressure generation is limited by the manometer designs which cannot generate pressures above 30 atm. There is also a theoretical risk of balloon rupture (rare but always possible). Trerotola found a way round the limits of current manometers by substituting them with a 1-mL polycarbonate syringe which can manually generate pressures above 30 atm in Conquest balloons [16]. Conquest balloons, however, have three shortcomings on top of their high price: they are rigid and poorly flexible, take a longer time to deflate, and their long shoulder makes them unsuitable for dilating anastomotic stenoses in forearm fistulas since the tip often encroaches into the distal radial artery. They only come in 5- to 12-mm diameter sizes. Their larger 14- to 16-mm diameter size balloons are called Atlas® and the smaller 4-mm version Dorado® from the same manufacturer. These latter two Conquest-derived balloons, unlike Conquest, are less able to take on ultrahigh pressure. A new UHP balloon, Mustang®, from Boston Scientific, gives as good a result as Conquest in the 3- to 7-mm diameter category. Mustang however cannot tolerate inflation pressures above 30 atm for balloons sized above 7 mm. Its advantage over Conquest is that it is extremely flexible and hence the dilation balloon of choice for stenoses located at acute angles like the arteriovenous anastomosis.
10.1.5.6 Selecting Balloon Diameter
The size (diameter) of the balloon selected for a particular dilation is based on both the location of the stenosis and the access vintage. Arterial stenoses in the subclavian artery require 7- to 9-mm balloons, while those in the axillary artery 7–8 mm, brachial artery 6–7 mm, upper forearm radial artery 5–6 mm, lower forearm radial artery 4–5 mm, and ulnar artery 3–4 mm.
Venous stenoses in forearm cephalic and basilic veins need by standard 6-mm balloons near arteriovenous anastomoses of new AVFs and up to 9 mm in older AVFs and repeat cases. At cannulation and aneurysmal segments of forearm, AVFs balloons as large as 12 mm may be used.
Cephalic and basilic vein stenoses near the anastomoses of upper arm AVFs, as a precaution, are dilated with 6-mm balloons on first dilation. At cannulation and transposed segments of upper arm AVFs, the starting balloon size is 7 mm, and this can go up to 12 mm in older AVFs. Cephalic arch stenoses require 7-mm balloons in recent accesses though this can be increased to a maximum of 12 mm depending on the age of the accesses.
Subclavian vein stenoses are dilated with 10- to 14-mm balloons, while brachiocephalic vein stenoses require 12- to16-mm balloons.
10.1.5.7 Balloon Rupture
Balloon rupture can occur at inflation pressures lower than the stated RBP as a result of manufacturing defects or when the balloon is trapped and damaged by intraluminal calcification seen more commonly in arteries than veins. It becomes a more frequent event once inflation pressure surpasses the RBP. In practice, this index is systematically underestimated for legal reasons as most balloons can tolerate pressures which are 50 % above their RBP (27 atm against a stated RBP of 18 atm for instance) though this goes totally against the manufacturers’ recommendation.
On first notice of balloon rupture, all efforts are made to deflate the balloon to the maximum (blood comes into the manometer), so it can be removed with ease over a guidewire through the introducer-sheath. If any difficulty is encountered with pulling out the balloon, the whole ensemble of balloon and introducer-sheath should be removed over a guidewire. A new introducer-sheath is then inserted over the wire. The ruptured balloon should be carefully inspected to make sure the fragments have been removed in toto and no pieces remain inside the vessel. There are reported cases whereby the distal piece of the balloon dislodged and embolized. The balloon tip is radiolucent and is therefore difficult to locate on angiography or fluoroscopy unless it is still lodged onto the guidewire from where attempts can be made to remove it with a goose neck snare (Fig. 10.15a–d). It is however futile and risky trying to retrieve it if it has already embolized into the pulmonary arteries where most venous emboli end and are unlikely to be of a major clinical risk, that is, pulmonary infarction or infection.
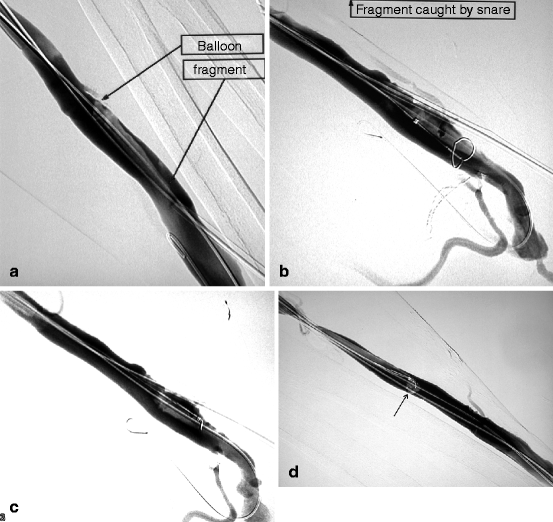

Fig. 10.15
(a) From a retrograde venous puncture of this left radial–cephalic fistula, the proximal radial artery was dilated with a 4-mm balloon. The balloon ruptured at an inflation pressure of only 8 atm, probably as a result of sharp luminal calcification. On withdrawal, it was realized that a fragment had remained in the vein, an exceptional event indeed. Luckily, on angiography, the balloon fragment could be located and as it was still lodged over the guidewire. (b) The 6-F introducer-sheath was exchanged over guidewire for a 9-F size through which a goose neck snare was pushed in contact with the balloon fragment. (c) Once the fragment was caught and secured within the snare, it was pulled toward the introducer-sheath. (d) As the balloon fragment could not be pulled through the introducer-sheath and coiled up at its tip (arrow), the whole ensemble of balloon fragment, snare, and introducer-sheath but not guidewire was removed in toto through the skin. The balloon fragments were inspected to ensure all pieces had been removed
Balloon rupture can cause a small puncture injury in the vein wall which can convert to a frank venous rupture. This type of venous rupture should be managed the usual way with prolonged balloon tamponade and occasionally stenting.
Deliberate balloon rupture is rather uncommonly induced when it becomes impossible to deflate the balloon. A fine needle is used to gently poke the balloon through the skin under fluoroscopic guidance.
10.1.5.8 Resistant Stenoses
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree