Early evaluation of patients with suspected CD
Evaluation of site and extension of CD
Differential diagnosis between chronic inflammatory colitis
Diagnosis of CD abdominal complications
Stenosis and intestinal occlusion
Internal and external fistulae
Intra-abdominal abscesses
Perforation and toxic megacolon
Assessment of CD activity
Post-operative follow-up and prediction of CD recurrence
2 Pathological Features
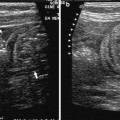
Crohn’s disease is a chronic granulomatous inflammatory condition of the alimentary tract of unknown origin. Although there are no pathognomonic features of the disease, it is characterised by a segmental and transmural inflammation of the intestinal wall, particularly ileum (30 %), colon (20 %) or both the large and small bowel (50 %). Macroscopically, the bowel wall often appears greatly thickened, and hypo-elastic or stiff with luminal narrowing (Fig. 1).
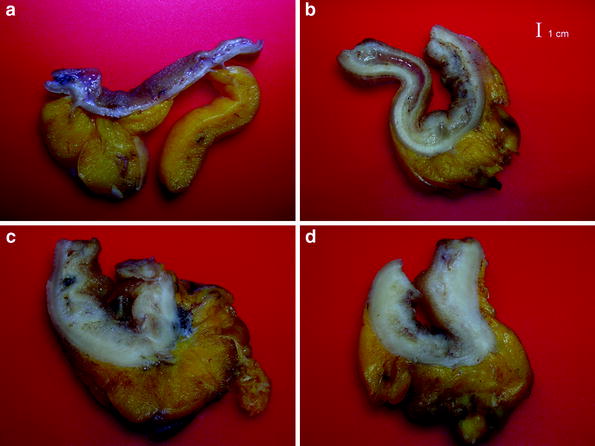

Fig. 1
Opened transverse sections of a normal ileal wall (a); thickened bowel wall in a CD patient with loose (left side) and markedly inflamed (right side) submucosa (b); markedly thickened bowel wall with fibrosis and loss of stratification (c,d). (Courtesy by dr. Paolo Fociani, Department of Pathology, ‘L. Sacco’ University Hospital, Milan)
Mucosal abnormalities consist of longitudinal and aphtous ulcerations, which, in advanced disease, may penetrate into the submucosa and muscularis leading the serosa and outside, creating fissures and fistulas. These may invade the adjacent loops, organs, skin or end blindly in the mesentery, sometimes resulting in intra-abdominal or retroperitoneal abscesses. The mesentery is often thickened and fatty, surrounding the thickened walls and containing enlarged lymph nodes.
3 Ultrasonographic Features of Bowel Walls
The abnormalities of bowel wall include bowel wall thickening, alterations of bowel wall echopattern, hyperemia, loss of elasticity and peristalsis, creeping fat and mesenteric lymph nodes. Intra-abdominal complications of CD typically include stenoses and obstruction, fissures and fistulae, inflammatory masses (phlegmon or abscesses).
3.1 Bowel Wall Thickening
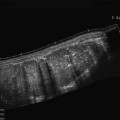
US examination in CD patients frequently reveals stiff and thickened bowel walls, usually >4 mm (which is considered the limit of normal bowel wall for the ileum and colon) up to 15 mm. The wall thickness of a diseased segment is measured in a transverse section from the central hyperechoic line of the lumen (representing interface between content of the lumen and the mucosa) to the outer hyperechoic margin of the wall (representing the serosa) (Fig. 2). Literature usually considers the maximum bowel wall thickness, that can be reproduced along the bowel wall for at least 2–4 cm.
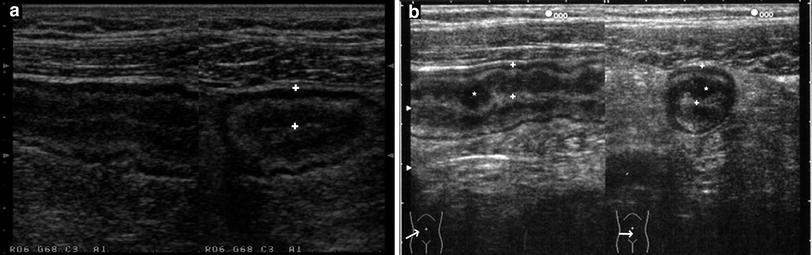

Fig. 2
a, b Longitudinal (left) and transverse (right) sections of a thickened bowel wall characterised by stratified echopattern showing the markers (+) on the inner hyperechoic line (lumen) and outer hyperechoic margin (serosa) of the thickened wall. (* = gross nodularity of the mucosa)
The abnormal thickening of bowel walls is the most widely and commonly US finding reported in the literature to diagnose CD. In a meta analysis aiming at evaluating the impact of different cut-off values of bowel wall thickening (3 vs 4 mm) in determining the presence of CD, it has been shown that when a >3-mm cut-off level was observed for abnormality, sensitivity and specificity were 88 and 93 %, respectively, whilst when a cut-off level of >4 mm was used sensitivity was 75 % and specificity 97 % (Fraquelli et al. 2005). The accuracy of US in detecting CD has been also compared with that of other cross-sectional imaging techniques, in a systematic review. This study showed that US has high accuracy for evaluation of suspected CD (overall per-patient sensitivity and specificity: 85 and 98 %, respectively), comparable to that of magnetic resonance imaging (MRI) (overall per-patient sensitivity and specificity: 78 and 85 %, respectively) (Panes et al. 2011).
However, these remarkable data also show that intestinal US, even in expert hands, may result in false-negative and false-positive findings. False-negative findings may be in obese patients or when CD is characterised by only superficial lesions, such as rare aphtous ulcers or mucosal erosions (Maconi et al. 1996a). False-positive findings rely on the fact that thickening of the bowel walls is not specific for CD, being present also in infectious, neoplastic and other inflammatory diseases (Truong et al. 1998). Therefore, when US is used as a first imaging diagnostic procedure, the diagnosis of CD is suggested when wall thickening involves the terminal ileum, is circumferential and segmental. However, the definitive diagnosis—when possible, and always for colonic lesions—should rely on endoscopic and histological examinations of pathological tissues. US may represent a useful tool, prior to other invasive or expensive diagnostic investigations, which can be postponed in the case of negative US findings.
In CD patients, US can be usefully employed in localising CD lesions within the bowel (particularly ileal lesions, which can be detected in more than 90 %) and in assessing the length of small bowel involvement (Brignola et al. 1993; Maconi et al. 1996a; Parente et al. 2003, 2004a, 2011).
The clinical significance of the degree of thickening of diseased bowel wall in CD is controversial. Several studies attempted to establish a relationship between maximum bowel wall thickness and clinical severity (Crohn’s disease activity index, CDAI) and biochemical activity (Erythrocyte Sedimentation Rate, C Reactive Protein) of CD. However, almost all the results of these studies produced weak correlations, although somewhat significant (Maconi et al. 1996a; Futagami et al. 1999; Mayer et al. 2000; Bru et al. 2001; Haber et al. 2000, 2002; Parente et al. 2003). On the contrary, the thickening of bowel wall significantly correlates with endoscopic activity of CD. It has been shown that US thickening of bowel wall has an overall high sensitivity and specificity (85 and 91 %, respectively) for the detection of endoscopic active CD (Panes et al. 2011).
Likewise, the US assessment of bowel wall thickening is useful to identify postoperative recurrences following resection, to assess the efficacy of conservative surgical treatment, and to obtain predictive data on the risk of recurrences in these patients. Postoperative endoscopic recurrences of CD may be correctly identified using bowel US in more than 80 % of patients (Di Candio et al. 1986; Andreoli et al. 1998). Moreover, US offers the possibility to assess the behaviour of diseased bowel walls following conservative surgery (namely strictureplasty and miniresection), in CD patients. In fact, in patients showing an improvement or return to normality of the bowel wall thickening 6 months after conservative surgery, the clinical and surgical recurrence rate have being significantly lower than in those maintaining the same level of bowel wall thickening (Maconi et al. 2001). Likewise, it has been shown that a high bowel wall thickness (>7 mm) at US is a major risk factor for intestinal resection (Rigazio et al. 2009; Castiglione et al. 2004).
Therefore, bowel US offers a useful alternative to invasive procedures in the postsurgical follow-up of CD patients, in particular for those CD patients who have undergone conservative surgery in whom endoscopy is not suitable to identify CD patients at high risk of clinical and surgical relapse, thus offering the opportunity to tailor the appropriate postoperative medical treatment.
3.2 Bowel Wall Echopattern
The thickened bowel wall in CD may show different echopattern. It may maintain the regular stratification (Fig. 2) or may be characterised by a partial or complete loss of layering. Sometimes, bowel segments with alternate persistence and loss of stratification, may be observed.
In stratified echopattern, the thickened walls display a variable enlargement of the mucosal, submucosal or muscular layers. Often the layer corresponding to the submucosa is thicker than others (Fig. 3a). The echo-stratification may be interrupted by hypoechoic areas (some with hyperechoic spots) corresponding to deep ulcers (Fig. 3b), or may completely disappear (Fig. 3c). The disappearance of echo-stratification indicates the presence of large, deep longitudinal ulcers associated with intense inflammation and neovascularisation (Kunihiro et al. 2004).
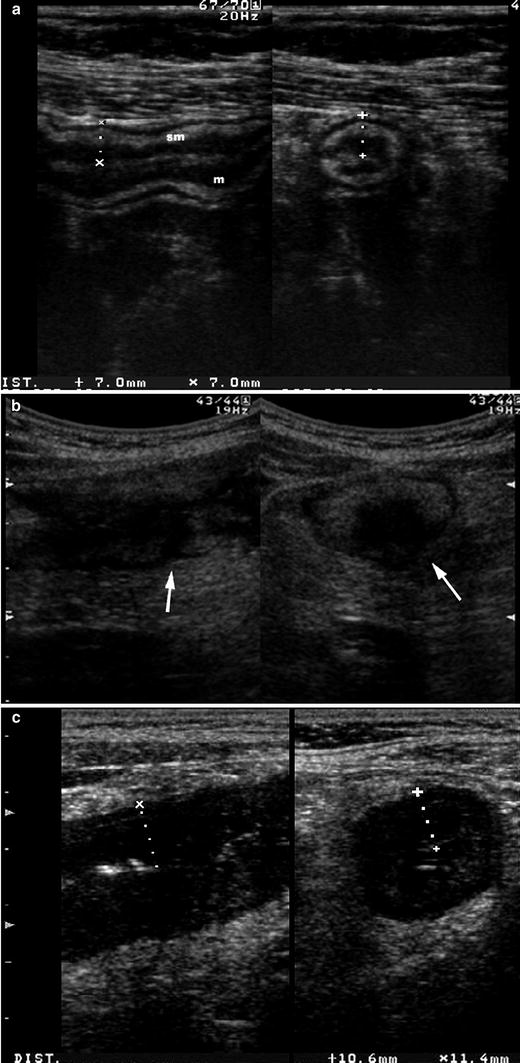

Fig. 3
Echopattern of thickened bowel walls. a Longitudinal (left) and transverse (right) sections of thickened bowel walls characterised by stratified echopattern with thickening of mucosal (m) and submucosal (sm) layers. b Longitudinal (left) and transverse (right) sections of thickened bowel walls characterised by stratified echopattern interrupted at the mesenteric side by hypoechoic areas (arrows), suggesting the presence of deep ulcers. c Longitudinal (left) and transverse (right) sections of thickened bowel walls characterised by hypoechoic echopattern with disappearance of wall stratification
The clinical and pathological significance of bowel echopattern and, in particular, its importance in defining CD activity has been investigated in two studies, one of which in patients with stenosis. In vitro studies, that compared US images with the in vitro histopathological findings of related resected specimens, showed that the loss of stratification (hypoechoic echopattern) correlated with the severity of inflammation and that persistence of stratification in bowel wall of strictures suggested a high degree of fibrosis within the submucosa and muscularis mucosae (Hata et al. 1994; Maconi et al. 2003a). A recent study showed that fibrosis is correlated with an increase of echogenicity in the muscularis propria and a decrease of echogenicity in the submucosa (Nylund et al. 2008).
3.3 Bowel Wall Vascularity
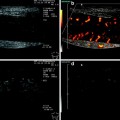
The increased vascularity is often observed in bowel wall with decreased echogenicity. This is likely due to hyperaemia and neovascularisation related to the increased inflammatory response (Maconi et al. 2003a; Di Sabatino et al. 2004). Therefore, vascularity within the thickened bowel walls, assessed by power-color Doppler US, has been used as an index of CD activity (Fig. 4).
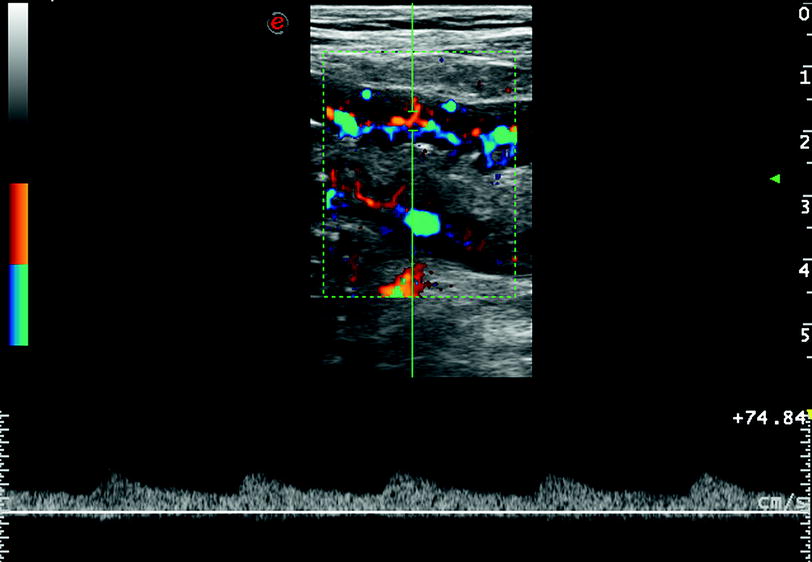

Fig. 4
Increased vascularity within the bowel wall showed by color Doppler ultrasound. Pulsed Doppler at this level detects the presence of low resistance arterial blood flow (bottom side)
Vascularity has been evaluated using a simple scoring system according to the semi-quantitative (and subjective) intensity of colour signals and/or by analysis of Doppler curves (measurement of resistive index) obtained from vessels detected within the bowel walls. However, neither of these parameters correlated well with clinical or biochemical activity in most studies, whereas often a significant correlation was often found between vascularity and endoscopical/radiological activity (Spalinger et al. 2000; Esteban et al. 2001; Haber et al. 2002; Heyne et al. 2002; Scholbach et al. 2004; Neye et al. 2004; Yekeler et al. 2005).
The vascularity of diseased bowel walls in CD has been recently assessed by US i.v. contrast agents. This issue will be widely discussed elsewhere (see “Intravenous contrast-enhanced bowel ultrasound”).
3.4 Elasticity and Peristalsis
Thickened bowel walls in CD may be associated with reduction or absence of peristalsis in the small bowel and loss of haustra coli in the colon (Sarrazin and Wilson 1996; Di Mizio et al. 2004). Although this manifestation is quite subjective and difficult to quantitatively assess, it has been regarded as relevant sign in most US studies, probably because associated with intestinal stenosis. Waiting for the results of US elastography (see “Imaging of Tissue Elasticity in Gastrointestinal Disorders”), oral contrast agents may be used for a more accurate US assessment of this feature (Parente et al. 2004b).
3.5 Mesenteric Hypertrophy and Mesenteric Lymph Nodes
Mesenteric hypertrophy (also named mesenteric fibrofatty proliferation or creeping fat) appears at US as hyperechoic, sometimes inhomogeneous area surrounding thickened bowel walls (Fig. 5). It is found in up to 50 % of CD patients (Goldberg et al. 1983; Maconi et al. 2008) and is correlated with biochemical and clinical activity of CD and with internal fistulas and increase bowel wall thickness (Maconi et al. 2008).
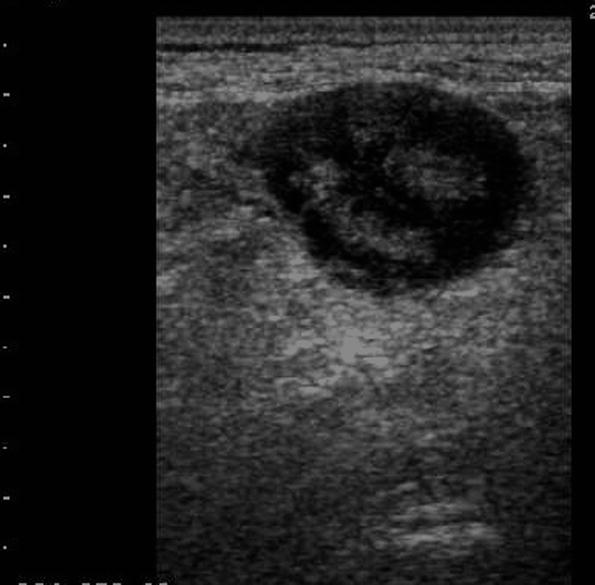

Fig. 5
Mesenteric hypertrophy, appearing at US as hyperechoic, sometimes inhomogeneous area surrounding thickened bowel walls
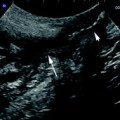
Enlarged mesenteric lymph nodes appear at US as oval, homogeneous hypoechoic nodules with regular margins (Fig. 6). This is a frequent finding in CD patients, in particular in young CD patients, in an earlier phase of CD and in patients with septic complications such as internal fistulas and abscesses. Its prevalence and clinical significance is discussed elsewhere in this book (see “Mesenteric Lymphadenopathy”).
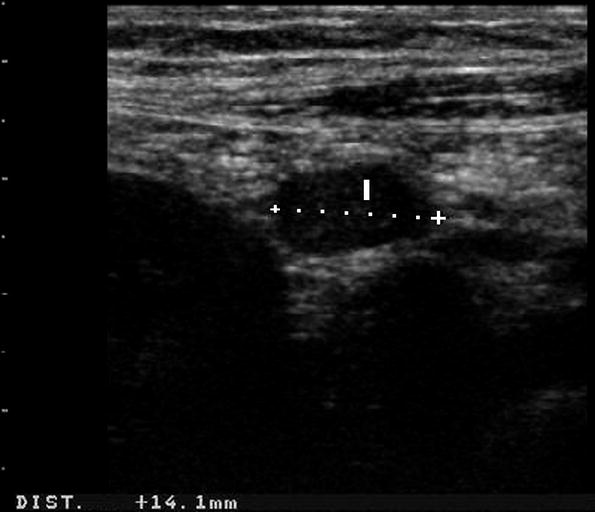

Fig. 6
Enlarged mesenteric lymph node (l) appearing at US as oval, homogeneous hypoechoic nodules with regular margins
4 Crohn’s Disease Abdominal Complications
The clinical course of CD is often characterised by abdominal complications such as stenosis, fistulae or abscesses and rarely by free perforation.
4.1 Stenosis and Intestinal Occlusion
Stenosis develops in 21 % of patients with ileal CD and in 8 % of those with ileocolic disease (Fenoglio-Preiser et al. 1989; Simpkins and Gore 1994). It is the most frequent cause of surgery. The diagnostic gold standard of this complication is contrast radiography that reveals all the occluded sites, the degree of intestinal narrowing and the length of the stenotic segments.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree