Aortic Anatomy, Physiology, and Function
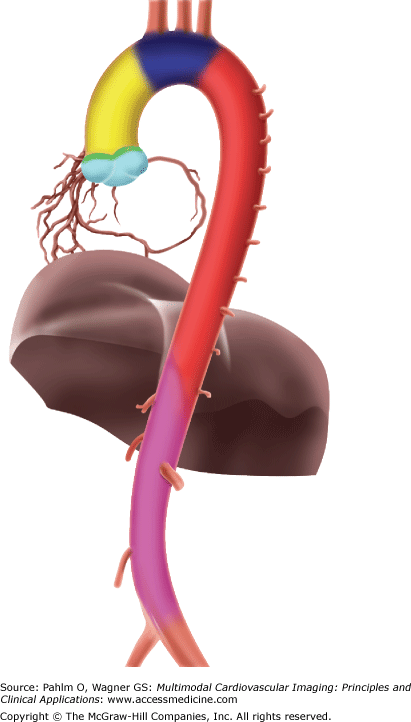
To have an understanding of aortic diseases, it is imperative to have a good knowledge base in anatomy, physiology, and function of the normal aorta. The aorta is a large conductance blood vessel, often called the greatest artery, normally reaching 0.7 m in length and 3.5 cm in diameter. It arises from the heart, arches anterocranially and then posterocaudally, and descends caudally, terminating as it bifurcates into the right and left common iliac arteries. The aorta is divided into six segments: (1) the aortic root, (2) the sinotubular junction, (3) the ascending aorta, (4) the aortic arch, (5) the isthmus and descending thoracic aorta, and (6) the abdominal aorta (Fig. 22–1).
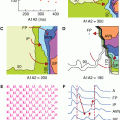
Figure 22–1.
Segmental division of the aorta: the aortic root (light blue), the sinotubular junction (green), the ascending aorta (yellow), the aortic arch (dark blue), the isthmus and descending (thoracic) aorta (red), and the abdominal aorta (purple). This figure was published in Aortic Diseases: Clinical Diagnostic Imaging Atlas, Hutchinson SJ, copyright Elsevier 2009.
The aortic root originates at the aortic valve annulus level and extends to the sinotubular junction. Normal diameter of the aortic root (at the widest proximal portion, known as bulbous) is up to 3.5 cm. The bulbous portion of the aorta is subdivided into the sinuses of Valsalva. The site where the bulbous portion of the aorta meets the narrower tubular-shaped aorta is termed sinotubular junction. Effacement of this junction suggests annuloaortic ectasia and often is seen in patients with Marfan syndrome. The ascending aorta follows the sinotubular junction and extends to the right brachiocephalic artery. The ascending aorta is an intrapericardial structure, measuring approximately 5 cm in length and up to 3 cm in diameter. The aortic arch is extrapericardial and courses leftward anterior to the trachea and continues posterior to the left of the trachea and esophagus and gives rise to all three great vessels: the innominate artery (brachiocephalic trunk), left common carotid artery, and left subclavian artery (Fig. 22–2). The normal diameter of the aortic arch is up to 2.9 cm. Arch branch vessel variants and right-sided arches can sometimes be seen. The isthmus is the narrower portion of the aorta (by approximately 3 mm) between the left subclavian artery and ligamentum arteriosus, a remnant of the ductus arteriosus. Blunt traumatic deceleration injury, resulting in transection to the aorta often occurs at this site. The descending thoracic aorta begins at the ligamentum arteriosus and continues to the level of the diaphragm. The descending thoracic aorta gives rise to intercostal, spinal, and bronchial arteries. The abdominal aorta starts at the hiatus of the diaphragm and courses retroperitoneal to its bifurcation. Major branch vessels include celiac arteries, superior and inferior mesenteric arteries, and middle suprarenal, renal, and testicular or ovarian arteries. The diameter of the suprarenal abdominal aorta is usually up to 2 cm. The infrarenal aorta diameter should never exceed that of the suprarenal aorta.
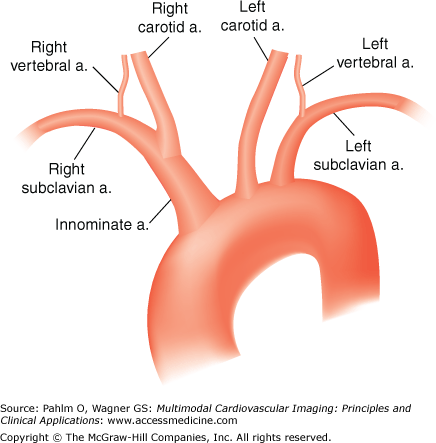
Figure 22–2.
The most common (normal) aortic arch branching pattern found in humans has separate origins for the innominate, left common carotid, and left subclavian arteries (a). Reproduced with permission from Layton KF, et al. Bovine aortic arch variant in humans: clarification of a common misnomer. Am J Neuroradiol. 2006;27:1541-1542, © by American Society of Neuroradiology.
The aorta has a trilaminar wall comprised of tunica intima, tunica media, and tunica adventitia. The tunica intima consists of an endothelium, subendothelial tissue, and an internal elastic membrane. The tunica media is composed of elastic fibers arranged as circumferential lamellae, elastin, collagen, smooth muscle cells, and ground substance. Adventitia is a thin, outermost layer of the aorta consisting of collagen; vasa vasorum, which is the vascular supply to the aorta; and nervi vascularis, which is the network of primarily adrenergic fibers.
Elastin-to-collagen ratio in the tunica media progressively decreases from proximal aorta to distal aorta and to peripheral arteries. This architecture ensures accommodation of stroke volume (cushioning function) and storage of potential energy in systole (capacitor function) and release of kinetic energy in diastole, propelling blood forward (Windkessel phenomenon). In addition, this is responsible for progressive increase in pulse wave velocity of propagation from the proximal aorta to peripheral vessels. In the normal aorta, the pulse wave is transmitted down the aorta to its bifurcation and peripheral vasculature where it is reflected back toward the heart during diastole, contributing to diastolic pressure. These properties usually decline with aging or diseased aorta due to disruption of elastin and decreased elastin-to-collagen ratio. In the diseased aorta, the propagation wave is faster, which causes an earlier return of the reflected waves (during systole) and a summation with the systolic wave. This leads to self-perpetuation of hypertension. In addition, increased aortic stiffness has been associated with increased risk of myocardial infarction, stroke, congestive heart failure, and both cardiovascular and overall mortality.
Aortic pressure waveform can be distinguished by its four components: (1) the upslope, (2) the peak, (3) the dicrotic notch, also known as incisura, and (4) the pressure decay. The slope of the upslope and the peak are dependent on the elasticity of the vessel and the volume of blood entering the aorta. Dicrotic notch is only seen in the proximal aorta and results from the aortic valve closure. The pressure decay corresponds to diastolic pressure and is affected by peripheral resistance and reflected pressure waves (in the healthy aorta). There is a progressive decrease in mean aortic and diastolic pressures and an increase in systolic aortic pressure with increasing distance from the heart.
The aortic “knob” may be seen in the superior mediastinum, on the left side, just lateral to spinal column on the chest roentgenogram. The aortic root and proximal ascending aorta are visible as they arise from the base of the heart on the lateral chest roentgenogram. Left anterior oblique projection provides the best view of the ascending aorta and the arch. Intimal calcifications and aneurysmal dilation may be seen.
Advantages of chest radiography include wide availability, no contrast exposure, low cost, minimal radiation exposure, and the ability to be performed quickly at the bedside. Disadvantages include low spatial resolution, two-dimensional (2D) static image, and relatively poor target acquisition for aortic diseases (Table 22–1).
| Variables | Chest Radiography | Echocardiography | Angiography | CT Angiography | MR Angiography |
|---|---|---|---|---|---|
| Spatial resolution | 1-2 mm | ~0.5-2 mm | 0.16 mm | 0.5-0.625 mm | 1-2 mm |
| Temporal resolution | N/A | 20-30 ms (M-mode <5 ms) | 1-10 ms | 83-135 ms | 20-50 ms |
| Contrast resolution | Moderate | Low-moderate | Moderate | Low-moderate | High |
| Radiation | 0.1 mSv | No | 5-8 mSv | 5.3-20 mSv | No |
| Target acquisition | + | ++ | +++ | +++ | +++ |
| Contrast exposure | No | No | Yes | Yes | No |
| Time | <1 min | 5-30 min | 30-60 min | <1 min | 30-40 min |
| Dimension | 2D | 2D or 3D | 2D | 3D | 3D |
| Availability | +++ | +++ | + | ++ | + |
The aortic root, sinuses of Valsalva, sinotubular junction, and proximal ascending aorta are usually well seen on a parasternal long axis view by transthoracic echocardiography (TTE).1 However, mechanical and stented bioprosthetic aortic valves produce acoustic shadows and reverberations. Distal ascending aorta and the arch are less well visualized by TTE. Suprasternal notch and supraclavicular windows offer the best views of the aorta in long and short axes. TTE allows visualization of the retrocardiac aorta in short and long axis (with clockwise rotation and lateral angulation of the transducer) on parasternal long axis view. Lateral angulation of the image plane in the apical two-chamber view can also be used to image the descending thoracic aorta. A posterior chest wall approach is particularly useful for imaging of the descending aorta in patients with large left pleural effusion. In the subcostal window, a lateral angulation of the transducer in the inferior vena cava plane allows visualization of the proximal abdominal aorta.
Transesophageal echocardiography (TEE) at 30° to 45° and 120° to 140° rotation at mid to high esophageal level is particularly helpful and less artifactual in evaluation of the aortic root, sinuses of Valsalva, sinotubular junction, and proximal ascending aorta. Withdrawing the transducer in the esophagus in the esophageal long axis plane produces more cephalad images of the ascending aorta. Medial turning and inferior angulation of transesophageal transducer at the arch level provide superior detail of the aortic arch in the long axis plane. Transesophageal transducer maybe turned around (either direction) at 0° to obtain a short axis view of the descending aorta. Rotation of the image plane to 90° allows depiction of the aorta in the long axis view, and the left subclavian artery may be located in this plane.
Ultrasonography (US) is a useful modality for abdominal aorta examination. It is commonly used for aortic disease screening, sequential monitoring for the assessment of aortic size, and evaluation of branch vessel involvement.
Advantages of echocardiography and ultrasonography include superb temporal resolution (especially with M-mode imaging), three-dimensional (3D) imaging, ability to assess functional cardiac complications of aortic diseases, wide availability, no contrast or ionizing radiation exposure, relatively low cost, and ability to be performed quickly at the bedside. Disadvantages include suboptimal target acquisition, inability to image the entire aorta, and can be time consuming.
The aortic root, sinotubular junction, and ascending aorta have significant motion throughout the cardiac cycle, and thus are prone to significant motion artifact with non–electrocardiography (ECG)-gated imaging modalities. ECG gating is recommended when imaging these proximal structures by either computed tomography (CT) or magnetic resonance angiography (MRA). The aortic arch, isthmus, and descending aorta are subject to little motion artifact.
CT allows various postprocessing displays and generates very detailed images of the arterial wall and lumen with high spatial resolution. Pacemaker wires in the superior vena cava (SVC) and brachiocephalic vein, mechanical aortic valves, pericardial structures, and contrast dye concentrated in SVC may produce artifacts when the aortic root, ascending aorta, and aortic arch are imaged with CT. The prevalence of motion artifact on a 1-second scanning time CT is reported to be as high as 57%.2 Multidetector CT (MDCT) technology significantly reduces respiratory artifacts and improves temporal resolution due to reduction of scanning time to 0.5 seconds. However, non–ECG-gated MDCT was reported to produce motion artifacts in 91.9% of cases.3 Fujioka et al4 reported a prevalence of diagnosis nonhampering motion artifact in 6.7% of cases with ECG-gated MDCT. In the absence of spinal prosthesis, CT of descending aorta produces very few artifacts.
Advantages of CT include 3D imaging, superb spatial resolution, excellent temporal resolution, very fast scanning time (<1 minute), high sensitivity and specificity for the assessment of aortic disease, postprocessing reconstruction, and wide availability. Disadvantages include ionizing radiation and contrast exposure.
Magnetic resonance imaging (MRI) and MRA can accurately depict the aortic lumen and the wall with minimal artifacts. The artifacts that one can encounter, however, include signal dropout from metallic clips and mechanical valves; ghosting artifacts (encountered when images are acquired during systolic phases of the cardiac cycle); insufficient dose of contrast agent (results in low signal-to-noise ratio); wraparound artifact, usually in coronal plane (aliasing due to data sampled in a discrete rather than continuous fashion); and susceptibility artifacts from concentrated contrast agent in the nearby venous structures.
MRI and MRA offer the ability to assess the aorta in both a 2D and 3D format. The number of different pulse sequences can be used to assess function and anatomy. A typical evaluation for the aorta would begin with anatomic assessment with axial, sagittal, and coronal dark blood turbo spin-echo (TSE) pulse sequence covering the entire thoracic or abdominal aorta. The dark blood TSE sequence obtains all the data for the particular slice within one heartbeat and thus allows for multiple slices without requiring the patient to hold their breath. Once the anatomic imaging is performed, cine images with a gradient echo pulse sequence are performed to assess the function of the aorta as well as the aortic valve (AV) when necessary. This image is generally performed in an oblique plane by scouting off of the dark blood TSE images. The next step in the evaluation includes typically a contrast-enhanced angiogram, which is gated to the heart (this avoids motion artifact within the proximal ascending aorta). The angiogram is a breath-hold sequence, which typically takes approximately 15 to 20 seconds to complete. If necessary, when blood flow needs to be quantified (ie, coarctation of the aorta), velocity-encoded pulse sequence is performed to assess the velocity of blood through the aorta at the level in question. This is the standard exam for the assessment of the aorta and can be tailored for each specific disease. The TSE dark blood sequence is useful in assessing the aortic wall, aortic dissection (AD), and other anatomic structures/abnormalities. If higher resolution is needed, then a single slice breath-hold TSE could be performed. This is often necessary when one wants to assess the aortic wall as in intramural hematoma or plaque assessment. The cine imaging is useful for more functional aspects of the aorta as in coarctation, AV insufficiency, and aortic distensibility. The MRA allows for a very high-resolution image of the aorta and is excellent in AD assessment and the 3D assessment of aneurysms. The benefit of 3D imaging is that the true cross-sectional diameter of an aneurysm can be easily assessed via postprocessing computer software.
Advantages of MRI include excellent temporal and spatial resolution, the wide spectrum of pulse sequences allowing for a 3D comprehensive assessment of the aorta, excellent target acquisition, and no ionizing radiation or contrast exposure. Disadvantages include lack of access at some centers, longer scanning time (can be prohibitive in patients who are hemodynamically unstable), artifacts due to respiratory motion in patients who are unable to breath-hold), inability of patients with large body habitus to fit inside the scanner, contraindication in patients with metal implants (eg, pacemakers/defibrillators, cerebral aneurysm clips), and the possibility that some patients may experience claustrophobia.
Aortography is an invasive test during which a multiholed catheter (usually pigtail) is passed percutaneously via radial, brachial, axillary, or femoral approach into the aorta; contrast material is injected via a power injector; and multiple radiographic views (or cine) are recorded. The ascending aortogram is often performed in a moderately steep, 30° to 60° left anterior oblique view. In the past, aortography was a first-line diagnostic test for imaging the aorta; presently, it is typically reserved for guiding aortic interventions.
Advantages of aortography include superb spatial resolution, excellent target acquisition, and good temporal resolution. Disadvantages include invasiveness, exposure to ionizing radiation and contrast, lack of access in some centers, production of 2D images, and time consuming to perform.
Anatomic Variants and Congenital Anomalies
In approximately 1% of healthy individuals, the AV is bicuspid forming two sinuses, rather than the usual tricuspid valve with three sinuses. Quadricuspid AVs with four sinuses are a rare finding with incidence of up to 0.01%. Bicuspid AVs may be associated with various pathologies of the ascending aorta and may result in AD. Anomalies of coronary artery ostia are common. These include separate ostia for left anterior descending artery and left circumflex artery, one or more infundibular (conal) arteries arising from separate ostia, duplicated arteries, high take-off (above sinotubular junction), and ostium arising from opposite sinus or pulmonary trunk.
The second most common pattern of the aortic arch is a common origin of innominate and left common carotid arteries, and a less common variant occurs when the left common carotid artery originates directly from the innominate artery. Both are commonly referred to as bovine-type arch. However, a true bovine aortic arch has no resemblance to any of these variations. In cattle, a single brachiocephalic trunk originates from the aortic arch and gives rise to both subclavian arteries and a bicarotid trunk, which then branches into right and left common carotid arteries. Other variants include internal mammary artery originating from the thyrocervical trunk and left vertebral artery coming off the aortic arch. Kommerell’s diverticulum is a diverticulum of the proximal portion of an aberrant left subclavian artery (occurs with right-sided aortic arch) or right subclavian artery (occurs with left-sided aortic arch), potentially compressing the esophagus posteriorly. Double aortic arch is a common form of vascular ring in which the trachea and esophagus are encircled by the aortic arches (right arch tends to be larger). The most common form of right-sided aortic arch is associated with an aberrant origin of the left subclavian artery, diverticulum of Kommerell, and left-sided ligamentum arteriosum. The second most common right-sided aortic arch is associated with brachiocephalic vessels originating from the arch in mirror-image fashion with the left innominate artery. The other two types are rare. A cervical aortic arch is a rare entity, sometimes associated with other cervical vessel anomalies and commonly presenting as a pulsatile mass on either side of the neck or supraclavicular area. Aortic spindle is a circumferential bulge in the posterior aortic arch area just distal to the left subclavian artery. Pseudocoarctation develops from elongation of the aortic arch and kinking at the level of insertion of the ligamentum arteriosum without a pressure gradient across this region (Fig. 22–3). Other very rare anomalies include double dorsal aorta, subclavian artery as the first branch of the aortic arch, persistence in the fifth aortic arch, and pulmonary artery sling. The great anterior radiculomedullary artery (the artery of Adamkiewicz) is the dominant feeder of the spinal cord. Therefore, in patients with thoracoabdominal aortic aneurysms, identification of this artery is critical to minimize the risk of postoperative spinal cord ischemia. The artery of Adamkiewicz originates from a left intercostal or lumbar artery, but in up to 75% of individuals, it arises from T9 to T12 intercostal artery. Ductus diverticulum is a focal, smooth convexity in the ventromedial aspect of proximal descending aorta. The important clinical significance of this structure lies in distinguishing it from a traumatic aortic disruption.
Aortic Dissection
Acute AD is an uncommon but catastrophic aortic disease. AD may frequently be overlooked, with the diagnosis in a significant number of cases being established only postmortem. The estimated incidence is up to 3.5 per 100,000 per year, with at least 7000 cases per year in the United States.5 Although early mortality was estimated at 1% per hour for untreated cases, the survival may be dramatically improved with timely management. In 1996, the International Registry of Acute Aortic Dissection (IRAD) was established, with the mission to better understand the presentation, diagnosis, management, and outcomes of patients presenting with acute AD. To date, IRAD has enrolled more than 2000 patients from 26 institutions and enlightened the understanding of this very important disease.6
Among the risk factors of AD, the most common are hypertension, advanced age, vascular atherosclerosis, preexisting aortic aneurysms, iatrogenic during cardiac surgery and percutaneous procedures, pregnancy (usually peripartum period), bicuspid AV, direct blunt trauma, Marfan syndrome, Ehlers-Danlos syndrome, inflammatory diseases, and cocaine use.
AD usually begins with an intimal tear (spontaneous due to diseased aortic wall, known as cystic medial degeneration, or iatrogenic, as a complication of various percutaneous or surgical procedures), allowing luminal blood to penetrate the medial layer, dissecting it longitudinally and forming the false lumen. Typically, the dissection extends anterogradely to or beyond common iliac arteries. The false lumen is often lateral to the true lumen in the descending aorta; however, medial or spiral (barber pole) orientations are described. AD may also be initiated with the rupture of vasa vasorum, resulting in aortic hematoma. Occasionally, the hematoma may extend distally or rupture through the intima causing an intimal tear and dissection.
Several classifications of AD have been developed, two of which, Stanford and DeBakey, are based on the aortic segment involved (Fig. 22–4). Recently, the Svensson classification was proposed and is based on the underlying pathologic process (Table 22–2). Stanford and DeBakey classifications share the same principle of grouping AD based on the involvement of ascending aorta (location of the intimal flap, irrespective of site of origin or location of the intimal tear), which in turn dictates the management and predicts the overall prognosis. ADs involving the ascending aorta comprise approximately two-thirds of AD, and current recommendations are to treat surgically, whereas ADs not involving the ascending aorta seem to do better with medical therapy (barring specific complications). Because the duration of AD (the time from symptom onset to presentation) also predicts the associated morbidity and mortality, AD is also classified as acute (<2 weeks) and chronic (≥2 weeks).
Figure 22–4.
DeBakey and Stanford classification systems for aortic dissection (refer to Table 22–2 for definitions). This figure was published in Braunwald’s Heart Disease: A Textbook of Cardiovascular Medicine, Libby P, et al, copyright Elsevier 2008.
| Type | Description |
|---|---|
| DeBakey | |
| Type I | Originates in the ascending aorta, propagates at least to the aortic arch and often beyond it distally |
| Type II | Originates in and confined to the ascending aorta |
| Type III | Originates in the descending aorta and extends distally down the aorta or, rarely, retrograde into the aortic arch and ascending aorta |
| Stanford | |
| Type A | All dissections involving the ascending aorta, regardless of the site of origin |
| Type B | All dissections not involving the ascending aorta |
| Svensson | |
| Class I | Classical aortic dissection with an intimal flap between true and false lumen |
| Class II | Medial disruption with formation of intramural hematoma |
| Class III | Discrete/subtle dissection without hematoma, eccentric bulge at tear site |
| Class IV | Plaque rupture leading to aortic ulceration, penetrating aortic atherosclerotic ulcer with surrounding hematoma, usually subadventitial |
| Class V | Iatrogenic and traumatic dissection |
The imaging techniques for AD center on the use of CT, TEE, MRI, and aortography. The choice of diagnostic modality depends on patients’ clinical status, equipment availability, and staff expertise. CT and TEE are the two most frequently used diagnostic tests, and the majority of the patients usually undergo two or more imaging tests (Fig. 22–5).
Figure 22–5.
Overall percentage of study patients according to the imaging study of choice. CT, computed tomography; MRI, magnetic resonance imaging; TEE, transesophageal echocardiography; TTE, transthoracic echocardiography. Reproduced with permission from Moore AG, et al. Choice of computed tomography, transesophageal echocardiography, magnetic resonance imaging, and aortography in acute aortic dissection: International Registry of Acute Aortic Dissection (IRAD). Am J Cardiol. 2002;89:1235-1238.
Spiral (helical) CT is the most frequently used imaging test for diagnosing acute AD around the world due to its accuracy and wide accessibility. CT provides a very detailed 3D depiction of the aorta and its branches. Earlier studies reported CT to be associated with insufficient sensitivity and specificity. However, a recent systematic review of the diagnostic accuracy of CT reported a mean sensitivity and specificity of 100% and 98%, respectively.7 When compared with TEE and MRI, CT was also better for ruling out AD in patients at low pretest probability.7 In addition to aiding in establishing the diagnosis of AD, CT assists in the management by providing invaluable information on the extent of the dissection, location of the intimal tear and flap, branch involvement, presence of an intramural hematoma, size of the true and false lumen, and presence of pericardial and/or pleural fluid (Fig. 22–6). Although motion artifacts of the aortic root and ascending aorta were rather common with older CT systems, contemporary 64-slice MDCT technology with ECG gating has significantly shortened scanning time, increased resolution, eliminated motion artifacts, and allowed simultaneous imaging of the aorta and coronary and pulmonary arteries.
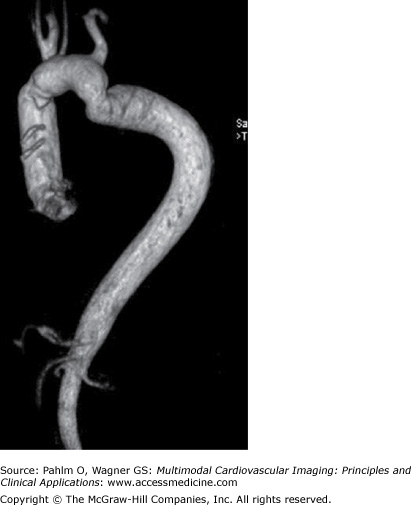
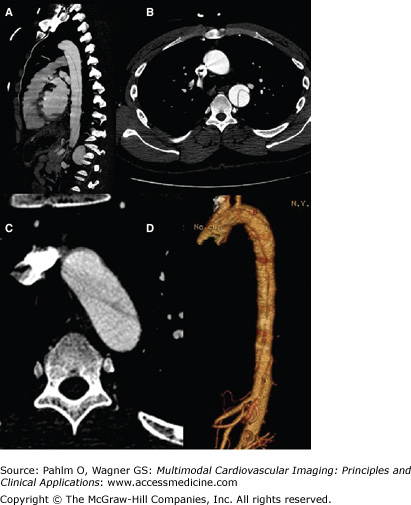
Figure 22–6.
Contrast-enhanced computed tomography scan showing type B aortic dissection originating at the aortic arch and terminating just superior to the renal arteries. Sagittal view (A), transverse view of the ascending and proximal descending aorta (B), magnified view of the aortic arch (C), and three-dimensional reconstruction (D).
Echocardiography is well suited not only for the diagnosis of AD, but most importantly, for the evaluation of cardiac complications of acute AD such as presence and severity of aortic regurgitation, identification of pericardial effusion and tamponade, and evaluation of regional left ventricular wall motion abnormalities (in case of extension of a dissection into the coronary arteries, usually the right coronary artery). TTE has a sensitivity of 59% to 85% and a specificity of 63% to 96% for the diagnosis of AD.8 TTE evaluation includes the standard and high parasternal windows for imaging of the ascending aorta, suprasternal notch window for the aortic arch, parasternal and apical windows for the descending aorta, and subcostal window for proximal abdominal aorta (Figs. 22–7 and 22–8).
Transthoracic suprasternal view showing the aortic arch and descending aorta.
Play Video
Figure 22–8.
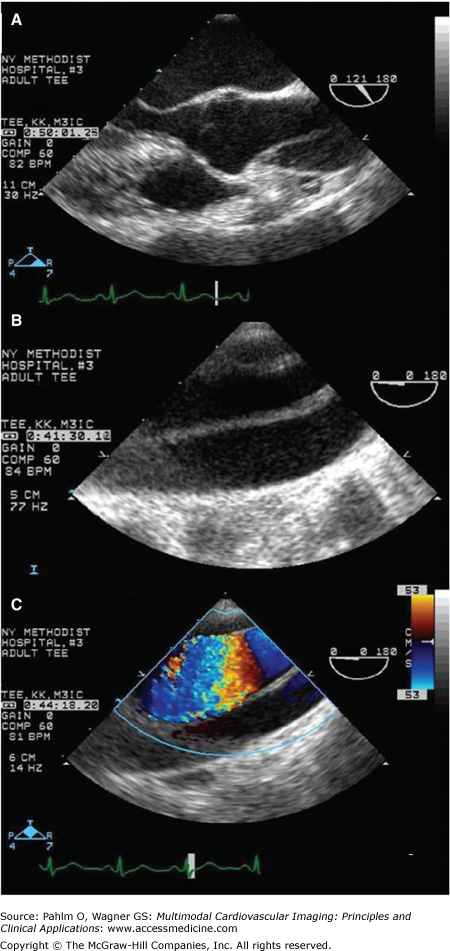
Transthoracic echocardiogram. Parasternal long axis view demonstrates massively dilated aorta (6.7 cm) compressing the left atrium (arrow). Type A aortic dissection with the true lumen (TL) separated from the false lumen (FL) by the intimal flap is evident. There is also evidence of the periaortic hematoma (H). LV, left ventricle.
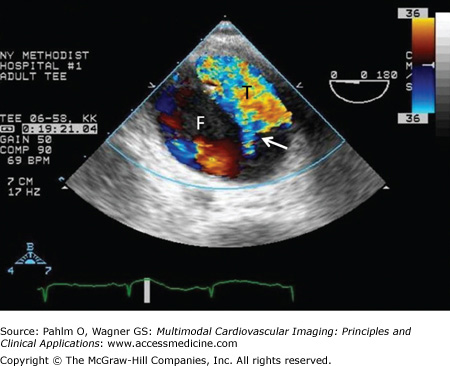
TEE is the second most commonly used imaging test in the diagnosis of acute AD. Anatomic proximity of the esophagus to the aorta makes it a far better technique than TTE, although proximal aortic arch is still difficult to image due to interference from the air-filled trachea and main stem bronchus (Fig. 22–9). The sensitivity of TEE for detecting AD is 98%, and the specificity is approximately 95%.7 Differential flow between true and false lumens can often be visualized (Fig. 22–10). TEE does not require intravenous contrast or radiation, and advantageously in hemodynamically unstable patients, it can be performed at the bedside in 10 to 15 minutes. However, TEE may be associated with bradycardia, aspiration, hypertension, hypotension, transient atrioventricular block, and rarely, esophageal perforation, especially in patients with esophageal disease (eg, strictures, varices).
Figure 22–9.
A transesophageal echocardiogram from a patient with acute type A aortic dissection. The intimal flap can be easily visualized in the long axis (120°) view of the aortic root and the ascending aorta (A) and in the long axis view (0°) of the aortic arch without (B) and with (C) Doppler color flow imaging.
The 2003 American College of Cardiology (ACC)/American Heart Association (AHA)/American Society of Echocardiography (ASE) practice guidelines for echocardiography give a class I recommendation for TEE use in patients with AD, aortic rupture, and follow-up of AD, especially after surgical repair when complication or progression is suspected (class IIa recommendation is given to follow-up of AD after surgical repair without suspicion of complication or progression). The practice guidelines give a class IIa recommendation for TTE use in AD and class IIb recommendation for aortic rupture.9
Briefly, the ACC/AHA/ASE classification of recommendations is as follows: class I—conditions for which there is evidence for and/or general agreement that a given procedure or treatment is beneficial, useful, and effective; class II—conditions for which there is conflicting evidence and/or a divergence of opinion about the usefulness/efficacy of a procedure or treatment; class IIa—weight of evidence/opinion is in favor of usefulness/efficacy; class IIb—usefulness/efficacy is less well established by evidence/opinion; and class III—conditions for which there is evidence and/or general agreement that a procedure/treatment is not useful/effective and in some cases may be harmful.
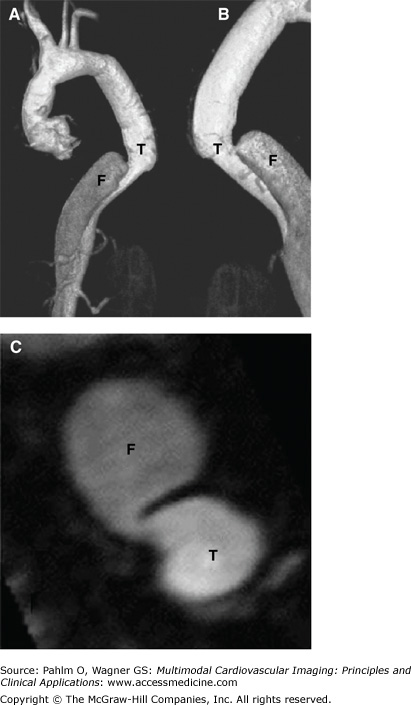
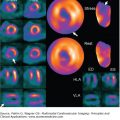
MRI and MRA have a high mean sensitivity and specificity (98%) and yield the highest value for confirming AD in patients with high pretest probability for AD.7 Despite this, MRI is much less commonly used in the diagnosis of acute AD (Fig. 22–11) because of its limited availability, time delay, incompatibility with implantable devices (MRI-safe permanent pacemakers are becoming more available) and surgical clips, limited monitoring during the study, and claustrophobia.
MRA with 3D reconstruction of a descending aortic dissection.
Play Video
Transaxial view revealing the connection between the true and false lumen.
Play Video
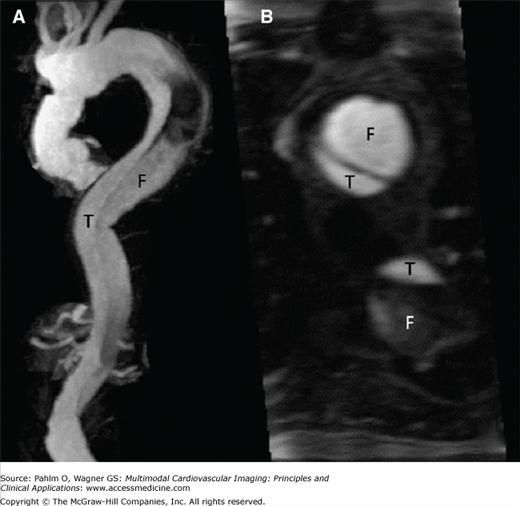
However, MRI has certain advantages. It is not associated with radiation exposure, has a high sensitivity for type A and type B acute AD, is useful in detecting features of intramural hematoma and penetrating aortic ulcer, and is considered the test of choice for follow-up evaluations of patients with surgically and/or medically managed aortic disease (Fig. 22–12). In addition, the safety profile of gadolinium is more favorable than that of the iodinated contrast medium used in CT, even though rare cases of nephrogenic systemic fibrosis in patients with severe renal insufficiency have been documented with gadolinium.10
Figure 22–12.
A. Magnetic resonance angiography of the thoracic and abdominal aorta in a patient with type A chronic aortic dissection who underwent surgery, showing the residual dissection that starts in the distal ascending thoracic aorta and involves the aortic arch and the descending thoracic aorta. B. Cross-sectional view of the distal ascending aorta and descending thoracic aorta, showing the dissection in these territories. Note the true (T) and false (F) lumen in the ascending and descending aorta. See Moving Image 22–12.