CLINICAL OVERVIEW
Cardiac imaging has a rapidly increasing role in the evaluation and management of patients with cardiac rhythm disorders. Atrial dysrhythmias are among the most common indications for cardiac imaging studies. Atrial fibrillation (AF) is a well-established risk factor for ischemic stroke, arterial thromboembolism, heart failure (HF), and dementia. AF is present in approximately 3% of the adult population. Imaging the left atrium (LA) plays a critical role in AF management. Ventricular dysrhythmias, although less common, are associated with significant morbidity and mortality in patients with heart disease. Scar tissue after myocardial infarction (MI) and in other cardiomyopathies is an arrhythmogenic substrate for the formation of ventricular tachycardia (VT).
DIAGNOSTIC STRATEGY AND THERAPEUTIC IMPLICATIONS
Atrial Dysrhythmias
Left Atrial Morphology and Function
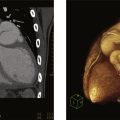
Among its other roles, the LA acts as a conduit and reservoir for blood returning to the heart from pulmonary circulation. Blood enters the LA posteriorly through the four pulmonary veins (PVs) and then flows anteriorly toward the mitral valve. The LA is separated from the right atrium by the interatrial septum (IAS), which is connected anteriorly to the aortic root ( Fig. 11.1 ). LA volumes can be measured by transthoracic echocardiography (TTE), cardiac computerized tomography (CCT) and cardiovascular magnetic resonance (CMR). Studies have shown that larger LA volumes are associated with AF recurrence after ablation. The left atrial appendage (LAA) arises from the LA and is an embryological remnant of the primordial LA. Its lateral border is the left lateral ridge, with infoldings of the atrial wall filled with external adipose tissue, separating the LAA from the left superior PV. The LAA is a long, lobed, tubular structure. The identification of the multiple lobes and their direction is paramount when imaging the LAA, both for preprocedural planning and intraprocedural guidance of LAA closure devices, and for ruling out or in the presence of an LAA thrombus. Four typical morphologies are used to describe the LAA: chicken wing (most common), windsock, cauliflower, and cactus ( Fig. 11.2 ). These can be identified in vivo by multiple imaging modalities (including CCT, CMR), transesophageal echocardiography (TEE), and angiography ( Fig. 11.3 ). These different shapes also correlate with varied stroke risks, as the cactus-shaped LAA most correlates with a history of stroke and the chicken wing shape with an absence of stroke history. The LAA is lined with pectinate muscles that form indentations along its inner surface. Occasionally, bulkier pectinate muscles can be confused with thrombi ( Fig. 11.4 ).




Left Atrial Thrombi
Patients with a history of AF are more likely to develop thrombus in the LA and LAA due to reduced contractility and increased stasis. This is often associated with reduced LAA emptying velocities on TEE Doppler studies. The LAA normally contracts in patients in sinus rhythm, however, patients with significantly elevated LV end-diastolic pressure may also be at risk for LAA thrombus formation, despite remaining in sinus rhythm. TEE is highly accurate in the detection of LA thrombi with a sensitivity of 93% and specificity of 100% and is considered the imaging reference standard. Using multiplane 2-dimensional TEE, as well as tissue and spectral Doppler, the LA and LAA can be thoroughly examined ( Fig. 11.5 ). Multiple findings in the LAA can represent different stages of thrombus formation ( Figs. 11.4, 11.6, and 11.7 ). Ultrasound contrast agents may be administered during the procedure to differentiate spontaneous echo contrast (smoke) from LAA thrombus ( Fig. 11.8 ). The presence of spontaneous echo contrast correlates with LA thrombus and is associated with reduced blood velocities and increased LA size. An algorithm for using TEE before elective cardioversion is shown in Fig. 11.9 .





CCT and CMR are imaging modalities that can be used for the assessment of thrombus in the LA and LAA. When compared to TEE, CCT has a similar ability to detect LAA thrombus, with the caveat that only TEE has been validated with surgical or postmortem anatomical findings. CCT with delayed imaging after contrast injection ensures maximal contrast filling of the LAA and lowers false positive rates ( Figs. 11.10, 11.11 ).


CMR of the LA can provide prognostic information. Atrial fibrosis as demonstrated by late gadolinium enhancement (LGE) on CMR is associated with LAA thrombi. Higher degrees of LA LGE are also associated with an increased rate of recurrence of AF in the first year after ablation.
Tables 11.1 and 11.2 summarize the indications and compare the advantages and disadvantages of different imaging modalities for LAA assessment and considerations for using CCT versus TEE for ruling out thrombus.
| TEE | MDCT | CMR | |
|---|---|---|---|
| Sensitivity/specificity for LAA thrombi detection |
| 96%/92% | 67%/44% |
| Spatial resolution | 0.2–0.5 mm | 0.4 mm | 1–2 mm |
| Temporal resolution | 20–33 ms | 70–105 ms | 30–50 ms |
| 3D volume rendering | Yes (with 3D) | Yes | Yes |
| Contrast required | No a | Yes | No a |
| Ionizing radiation | No | Yes | No |
| Special considerations |
|
|
|
a Contrast may be used to enhance the visualization of a thrombus in equivocal cases.
| Indication | Incidence of LA/LAA Thrombus | Recommendation |
|---|---|---|
| Cardioversion | 2.9%/4.4% a,b to 13.8% c |
|
| Pulmonary vein isolation | 1.9%–5.4% c | CTA |
| Stroke evaluation |
| TEE |
| Atrial appendage occlusion | 6.3% | CTA + TEE |
a Prevalence of LA thrombus in this group is extrapolated from the prevalence in preablation cohort.
Guidance of Left Atrial Appendage Closure
Prevention of systemic emboli with anticoagulation therapy is one of the primary objectives in the treatment of AF. However, as not all patients can tolerate this treatment, an alternative is the closure or exclusion of the LAA, either percutaneously or surgically. The two devices available for percutaneous closure are the Watchman device (Boston Scientific) and the Amplatzer Cardiac Plug.
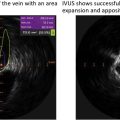
The essentials of preprocedural planning are ruling out an LAA thrombus, measuring LAA orifice and depth, and characterizing the LAA anatomy and morphology. These can be performed by either CCT or TEE ( Fig. 11.12 ). TEE provides intraprocedural image guidance and can be performed at the bedside without radiation or the use of contrast, while CCT is noninvasive and provides more accurate sizing. Detailed anatomical description of the LAA, including the presence of trabeculations, pectinates, angulations, and additional lobes is key to device sizing and selection. Using TEE, the diameter and depth of the LAA are measured in 4 omniplanes (0, 45, 90, and 135 degrees), and the course of the LAA should be described to assist with the optimal location of the transseptal puncture. The fossa ovalis, the thinnest part of the IAS, is used for transseptal puncture during structural heart procedures ( Fig. 11.13 ).


Intracardiac echocardiography (ICE) can be used during ablation and LAA closure procedures to provide real-time imaging. Its images can be integrated into an electroanatomical (EAM) mapping system. During atrial procedures, the ICE catheter primarily resides in the right atrium and can help guide transseptal puncture, visualize the origin of the PVs, and identify ablation targets. Importantly, ICE can help minimize complications of left atrial procedures by visualizing the proximity of the esophagus to ablation catheters and mitigate the morbidity of complications such as cardiac perforation by early identification of pericardial effusions.
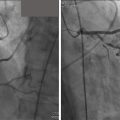
An alternative to percutaneous closure of the LAA is surgical exclusion ( Fig. 11.14 ). The LAA is cut to its base and the incision is closed. This is generally performed as an adjunct to cardiac surgery for another indication (e.g., coronary artery bypass grafting or valvular surgery), however, it can also be done as a separate, minimally invasive, or thoracoscopic procedure.