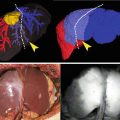
Fig. 9.1
Fluoroscopic view of the clip applier on the cystic duct. The clip applier is visible thanks to the intense reflected light from the liver
Intraoperative Cholangiogram (IOC)
The most commonly utilized technique to assess the intraoperative biliary anatomy is the intraoperative cholangiography (IOC). This technique consists in the direct injection of contrast media in the biliary tree (the gallbladder, cystic duct, or more rarely common bile duct) and visualization via real-time fluoroscopic imaging equipment. Extensive literature exists for both the routine and selective use of IOC. The proponents of routine IOC report a simpler utilization and learning curve for both the operator and the staff because of the consistency of its application. These factors contribute to the overall efficiency with which IOC can be performed in the reporting medical centers (average added time 7–10 min) [9]. Also the routine use allows for more confident interpretation of the images by the surgeon. In fact some of the criticisms of IOC is the possibility of misinterpreting the images obtained, obviating the benefits of the added cost and time. Although conflicting evidence exists, at least two cohort studies have shown a reduction in BDI when routine IOC is implemented. In fact, Flum et al. found a reduction of almost 50 % in the rate of BDI when IOC was routinely used [10]. Similar rates of reduction in BDI injury with the use of routine IOC were demonstrated by Fletcher et al. [11].
The cost of IOC remains a very controversial topic. In fact evidence of both the cost effectiveness of routine IOC and selective IOC exists. The proponents of routine IOC report costs of performing IOC of around $675 per case [5]. This number multiplied by the number of LC performed yearly results in an added cost to the operations of over $1,000,000,000. When the potential cost of BDI injury and its incidence are considered, however, routine IOC would result in savings estimated between $164,000,000 and $1,290,000,000 per year [5]. Obviously the cost advantage is only beneficial if calculated in large scale, whereas for the individual surgeon the time and money saved would outweigh the low probability of incurring in BDI. In addition, BDI is linked to high probability of litigation often resulting in large settlements and payouts (between $5,000,000 and over $6 million) [5]. Unfortunately, the calculations of costs are flowed by approximations and extrapolations. In fact, often the calculation of the cost of each IOC only includes the tangible expenses of the supplies utilized, not taking into consideration costs related to equipment’s depreciation, maintenance, OR time (estimated about $1,000/h), radiologist fees. Under this perspective it will require 714 IOC to prevent one BDI, which translates in a projected cost of $371,000 [12, 13].
One of the major criticisms to IOC is the basic concept of the technique itself. In fact, in order to perform an IOC the cystic duct has to be incised. This critical step is by definition the lowest degree of BDI (Bismuth 1) if the main bile duct is mistaken for the cystic. So even if the IOC is fundamental in order to recognize the anatomy, the chance of BDI is not diminished, but only recognized early. Undoubtedly, early recognition and repair of BDI has been linked with significantly better outcomes and cost saving [14]. Finally, the use of routine IOC is certainly superior in the diagnosis of CBD stones and results in decreased re-admission rates [14]. The latter factor should be accounted for in the cost analysis for routine IOC.
Cholecystocholangiography (CCC)
In order to obviate the opening of the biliary tree to insert the cholangiogram catheter, the technique of cholecystocholangiography (CCC) has been described. In the latter, the contrast is injected directly in the gallbladder and so higher pressure and quantity of contrast are required. In spite of the advantages of this technique in terms of avoidance of cystic duct cannulation and faster execution compared to the standard IOC, in randomized trial the image quality was inferior to the IOC and there was more than doubling of the radiation exposure [15]. An alternative to this technique is the injection of contrast directly at the infundibulum of the gallbladder using a particular occluding clamp (the “Kumar clamp”). The success rate of this technique has been reported in 80–90 % of the cases, but its superiority to the standard IOC still needs to be proven [16].
Laparoscopic Ultrasound (LUS)
The popularity of laparoscopic ultrasound (LUS) led to the implementation of such technique for the assessment of the biliary anatomy. In fact, LUS can visualize the CBD, the CBD–cystic duct junction and the vascular anatomy. Among the advantages of the technique are the lack of radiation exposure, the repeatability, and the short procedural time. The success rate in the visualization of the structures has been reported in over 90 % of the cases [17]. Also the cost is minimal since it only include the capital expense of laparoscopic probes that are already likely available as they serve multiple other applications in laparoscopy (liver, pancreatic surgery, etc.). Unfortunately, the procedure has never become popular among the general surgeons because of its steep learning curve in the interpretation of the images.
Fluorescent Cholangiography (FC)
The advantages of the use of fluorescence in surgery are determined by the possibility to obtain a high contrast image between the target structure and the background thanks to the different light wavelengths utilized to illuminate and record [18]. Additionally, the simplicity of the technique along with its safety and low cost makes it for an ideal technology for the expanding field of image-guided surgery.
Indocyanine green (ICG) was the first fluorescent dye described. This was initially utilized for near-infrared (NIR) photography in 1955 [18]. The first clinical application was in retinal angiography in the 1970s [19]. In order to visualize the blood vessels in a dark background, a proper light source is necessary to illuminate the ICG with wavelengths between 750 and 800 nm. The resulting fluorescence with wavelengths over 800 nm is captured using a camera with filters able to avoid the interference of the two light wavelengths, in particular the brighter excitation light. The ability to work in the NIR spectrum allows for more penetration into the tissues. Other substances like fluorescein, instead, are visible without the need of special cameras, but give information of more superficial layers.
The clinical applications of ICG imaging span from diagnostic modalities (such as tomography, retinal, cerebral, coronary angiographies) to therapeutic applications in lymphatic mapping, assessment of tissue perfusion, cancer identification and targeting. More recently ICG imaging has found a specific application in laparoscopy, by its property of visualize particular anatomic structure, allowing for more accurate and safe procedures.
The property of ICG to bind with plasma proteins allows for confinement into the vascular system. The binding to lipoproteins in particular is so rapid that the ICG can be safely re-administered in a short time interval [18]. The ICG is then rapidly, and almost exclusively, eliminated by the liver into the bile. The specific property of ICG to fluoresce at different wavelengths makes it for a potential specific molecular carrier. Unfortunately, once the ICG is conjugated to antibodies loses its fluorescence until is dissociated from them [18].
The ability of rapidly binding to plasma proteins after an intravenous bolus makes ICG an optimal substance to utilize in angiography. In fact, ICG angiography has been described in neurosurgery to verify complete obliteration of AV malformations and in general to evaluate the intracerebral circulation in real time at the time of surgery and in a reproducible manner. Also ICG angiography is utilized to assess patency of revascularization procedure such as coronary bypass grafting. Both the peak fluorescence intensity and the temporal slope of fluorescence in the tissue can help quantify tissue perfusion in a more objective manner. Similarly, the use of near-infrared spectroscopy (NIRS) can assess and quantify the degree of liver perfusion after injection of ICG [19, 20].
The overall safety profile of ICG is excellent. In fact, due to its minimal production of singlet oxygen, the potential for phototoxicity, of the retina in particular, is very low. Although in small amounts, ICG contains iodine and could generate allergic reaction in predisposed individuals. In alternative, a compound without iodine, infracyanine green (IfCG) has been developed. This is less than ten times retina toxic than ICG [18]. Because of its lack of metabolites ICG is readily excreted into the bile, which makes it the ideal substance for cholangiography. This property also limits the dose necessary to visualize the extrahepatic biliary tree to 0.5 mg/kg of body weight, well below the threshold of side effects described with doses above 5 mg/kg.
One of the main limitations of the use of ICG in cholangiography is related to the limited penetration of the tissues. In fact, the tissue optical window depends on the ability of the NIR light to reach the target tissue. The variable presence of connective tissue, worse in the presence of more intra-abdominal fat or acute inflammatory changes (edema), limits the utility of this methodology. In our experience of over 100 laparoscopic cholecystectomies with intraoperative fluorescent cholangiography, we were able to visualize at least one of the extrahepatic biliary structures in 100 % of the cases even in the presence of inflammation (unpublished data).
In order to obtain the best definition, the excitation light should be filtered so it does not mix with the fluorescent one. In order to do this the excitation light, especially if it is a broad spectrum one, should be filtered to block the longer wavelengths. In fact, the fluorescent light has only a small portion of the spectrum of the exciting light and it would not be able to be visualized without filtration. This problem is obviated if a monochromic light such as the laser is utilized. Since the normally used charge coupled device (CCD) sensor cameras are normally equipped with a filter that cuts the NIR wavelengths in order to reduce interference, special filters are then necessary to obtain the visualize the fluorescent image.
More recently, the utilization of fluorescence in surgery has led to the development of several techniques to visualize the bile ducts. As previously mentioned, ICG has the unique feature of completely binding to serum protein and exclusive excretion into the biliary tree. Initially, this property was utilized by injecting high ICG doses intravenously and the bile ducts could be identified without any special filters because of the dark blue discoloration [21]. It was soon obvious that the discoloration when observed at the visible light spectrum (380–600 nm) had a penetration of only few mm. This was impractical in many cases in which thick connective tissue or inflammation was present at the level of the infundibulum. In addition, the leak of such concentration of dye would obscure the anatomy and potentially impair the surgeon’s ability to perform a safe dissection. On the other hand, the significant advantage of lack of radiation, real time visualization, and, more importantly, the possibility of identify the extrahepatic biliary anatomy prior to any dissection/injury fomented the interest and research in this field [22].
At the same time, infrared cameras were developed and Liu et al. were able to delineate the extrahepatic anatomy by infusing saline into the biliary tract of pigs at different temperature than core and contrasting the image with the body temperature [23]. Obviously, this was an impractical technique because of the quick equilibration on the saline temperature and the need for repetitive injections.
It was in 2009 that Ishizawa et al. describe for the first time the use of ICG excited with NIR light and its fluorescence captured with a filtered camera for the purpose of obtaining visualization of the biliary tree anatomy [24]. In their series of 52 patients, Ishizawa et al. reported a 100 % visualization of the cystic duct and 96 % visualization of the cystic duct-common hepatic (CHD) junction prior to any dissection of Calot’s triangle [24]. Although they found that FC was helpful in determining the level of division of the cystic duct in order to avoid leaving cystic duct stones behind, the methodology is not helpful in identifying CBD stones [24].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree