Abstract
Objective
This retrospective study aimed to investigate the efficacy and safety of focused ultrasound (FU) treatment for high-risk human papillomavirus (HR-HPV) infection-related cervical intraepithelial neoplasia grade 2 (CIN2) in nulligravidae under 35 y old, while also assessing pregnancy outcomes post-treatment.
Methods
Nulligravid patients aged 18–35 y with histologically confirmed CIN2 and HR-HPV infection were included in the study. We collected demographics, pertinent medical history, HPV genotypes and cervical length at baseline. Follow-up evaluations were conducted at 6- and 12-mo intervals post-treatment to assess histopathological response, HPV infection clearance and adverse events related to treatment.
Results
A total of 31 eligible patients were recruited and underwent FU treatment. At the 6-mo follow-up, complete pathologic response was observed in 22 out of 31 patients (70.96%), while partial response was seen in eight out of 31 patients (25.80%). The average duration from pathological diagnosis to achieving either a complete response or partial response after treatment was 214.36 ± 24.31 (186–270) d. The baseline remission rate for HPV was 35.48% at 6 mo, increasing to 71.49% at 12 mo. Moderate lower abdominal pain and increased vaginal discharge were the most frequent adverse events. Among the patients desiring pregnancy, the successful pregnancy rate was 57.14%, resulting in eight deliveries.
Conclusion
FU demonstrated a favorable safety profile and efficacy in nulliparous females under 35 y old with CIN2, and its benefits for fertility warrant further investigation.
Introduction
There is a consensus that high-grade squamous intraepithelial lesions (HSIL)/cervical intraepithelial neoplasia 2 (CIN2) embodies a blend of biological characteristics from both CIN1 and CIN3, possibly influenced by variances in colposcopy biopsies and pathological interpretations [ ]. According to the World Health Organization Classification of Tumors, both CIN2 and CIN3 fall under the category of HSIL [ ], indicating the necessity for treatment. Surgical interventions such as cold-knife conization (CKC) or the loop electrosurgical excision procedure (LEEP) are typically recommended for treating HSIL/CIN2 [ ]. However, these procedures may elevate the risks of preterm birth and mid-trimester loss in women conceiving post-treatment; [ ] thus, avoiding unnecessary surgeries is crucial.
Studies have shown that approximately 50% of CIN2 cases naturally regress within 2 y, with 32% persisting and only 18% progressing [ ]. Notably, among women under 30, around 60% experience regression, 23% have persistent diseases and 11% progress [ ]. Recent guidelines from the American Society for Colposcopy and Cervical Pathology recommended follow-up for individuals under 25 with HSIL/CIN2, while treatment is favored for those over 25 unless concerns regarding future pregnancies outweigh cancer risks [ ]. However, positive results from HPV DNA tests, abnormal cytological findings, and colposcopy have been linked to varying degrees of psychological distress and financial strain over the follow-up period [ , ]. Thus, for women seeking to preserve fertility while addressing HSIL/CIN2, a non-invasive therapeutic approach that minimizes the risk of progression to CIN3 or carcinoma in situ is preferred.
Focused ultrasound (FU) has emerged as an innovative, minimally invasive treatment modality that employs a specialized ultrasound transducer to precisely target specific anatomical and histological locations, spanning from deep to superficial layers while preserving the integrity of the gross cervical structure [ , ]. Through its ability to promote local immunity and eradicate high-risk human papillomavirus (HR-HPV) infection, FU holds promise for clearing HR-HPV infection and helping cervical lesions regress [ ]. Numerous studies have investigated the efficacy of FU in treating HSIL, with no observed adverse effects, including preterm birth, premature rupture of membranes or low birth weight infants [ , ]. With its minimal risk of severe complications and ability to precisely target tissues, FU is a valuable alternative for treating HSIL.
Despite its promising potential, the application of FU in nulligravid patients has been underexplored. Therefore, this study aimed to evaluate the efficacy and safety of treating cervical HSIL/CIN2 in nulligravid women with FU.
Material and methods
Patient selection
This study was approved by the Ethics Committee of the Affiliated Hospital of North Sichuan Medical College (Approval Code: 2023ER429-1). We adhered to the principles of the Declaration of Helsinki, as well as domestic and international standards. Patients with a diagnosis of HSIL/CIN2 and HR-HPV infection at the affiliated hospital of North Sichuan Medical College from January 2019 to December 2022 were identified. All charts were reviewed, and patient demographics and pertinent medical information were recorded.
The inclusion criteria were: (i) aged between 18 and 35 y old with a future conception plan; (ii) confirmed diagnosis of CIN2 on biopsies; (iii) tested positive for at least one HR-HPV; (iv) cervical lesions covering less than three quadrants with clear margins on colposcopy; (v) clear squamous-columnar junction; (vi) cytologic findings of negative intraepithelial lesions or malignancy (NILM), negative atypical squamous cells of undetermined significance (ASC-US), negative low-grade squamous intraepithelial lesions (LSIL) and HSIL.
The exclusion criteria included: (i) a history of abnormal cytologic findings indicating atypical squamous cells, ASC—cannot exclude high grade squamous intraepithelial lesion highly, or more severe lesions; (ii) previous cervical surgery, pelvic radiation or chemotherapy; (iii) a history of recurrent spontaneous abortion or preterm delivery; (iv) presence of organic diseases that could impact pregnancy outcomes; (v) acute inflammation of the reproductive system; (vi) histopathological grading necessitating additional treatment; (vii) combined immune system deficiency disorders; (viii) unsuccessful colposcopy or lesions in the cervical canal; (ix) patients needing adjuvant therapy or other treatment during follow-up; (x) a history of previous cervical procedures like conization or ablation; (xi) patients with recurrent HSIL; (xii) patients with severe cardiac, hepatic, renal, hematologic or autoimmune diseases; (xiii pregnant women or post-partum women who were breastfeeding; (xiv) patients who were using medications vaginally. Written informed consent was obtained from all participants.
FU treatment protocol
A Model-CZF300 FU therapeutic device (Chongqing Haifu Medical Technology Co., Ltd., China) was used. This device operated within a treatment force ranging from 3.5 to 4.5 W, at a frequency of 10.0 MHz, with pulses at 1000 Hz. The patient was placed in the lithotomy position, and a skilled gynecologist identified and exposed the cervix by using a sterile speculum. Following cervix disinfection, ultrasound coupling gel was applied, and the FU probe was then placed at the cervix. A circular scan was conducted from the lesions toward healthy tissue, extending approximately 2 mm beyond the lesion margin.
The criteria for treatment termination included: (i) the treatment area shrank and the external cervical os being slightly depressed inward and appearing slightly white; and (ii) negative results of staining post-treatment showing the cervix coated with a 1% Lugol’s iodine solution with negative staining results.
Post-treatment follow-up
After treatment, all patients were asked to strictly abstain from sexual intercourse for 2 mo and recommended to abstain for an additional 3 mo to minimize complications. Clinical assessment, including vaginal bleeding, wound infection, wound healing, cervical adhesions and scaring, was performed by a skilled gynecologist. Colposcopy and multi-point cervical biopsies were also performed. The HR-HPV genotyping test and ThinPrep cytologic test (TCT) were repeated every 6 mo. The cervical length was reassessed 3–6 mo post-surgery via transvaginal ultrasound, and then compared to the preoperative baseline data. Information regarding the time to conception and pregnancy outcomes following treatment was gathered through telephone interviews, with the final interview conducted on February 29, 2024.
TCT and HR-HPV subtypes
During the 6-mo follow-up period, cervical specimens were collected by using an endocervical brush. A TCT was performed for all samples, and the results were interpreted according to the Bethesda System terminology. An HPV genotyping real-time polymerase chain reaction kit (Shanghai ZJ Bio-Tech Co., Ltd., China) was used to identify 15 subtypes of HR-HPV DNA, including 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, 68 and 82.
Colposcopy and cervical biopsies
During the 6-mo follow-up period, colposcopy procedures were performed on all women who were enrolled in the study. The standard procedure comprised three sequential steps: initial cleansing of the vaginal and cervical surfaces using a saline solution; subsequent application of 3%–5% acetic acid to facilitate the detection of CIN; and, where warranted, utilization of Lugol’s iodine for a comprehensive examination. The interpretation of colposcopy images adhered to the 2011 International Federation for Cervical Pathology and Colposcopy terminology for colposcopy. Diagnoses were categorized as either normal, LSIL, HSIL or carcinoma based on the findings [ ].
After colposcopy, multi-point cervical biopsies were then performed, followed by histopathological examination. Suspicious areas identified during colposcopy were biopsied using biopsy forceps. In instances where no abnormalities were detected during colposcopy, biopsies were then systemically obtained from the squamous-columnar junction in four quadrants, along with an endocervical curettage. All collected samples were preserved in 10% formalin, processed with hematoxylin and eosin staining for microscopic examination and analyzed by experienced pathologists at our hospital. Histopathological results were categorized as normal, LSIL, HSIL or carcinoma according to the 2012 LAST guidelines [ ].
Definitions
The complete pathologic response of HSIL/CIN2 was identified by the absence of initial abnormalities and normal histology. The partial response was defined as histologic downgrading to LSIL or condyloma-like. Persistence of HSIL/CIN2 meant that no changes were observed during a colposcopy-directed biopsy. Progression was characterized by the detection of HSIL/CIN3 or cervical cancer on follow-up biopsy pathology.
Statistical analysis
Continuous variables with a normal distribution were expressed as the mean and standard deviation, while those deviating from normality were presented as the median and range. Categorical variables were expressed as frequencies and percentages. Pearson ’ s chi-square test and the Wilcoxon test were used to compare the differences, and all statistical analyses were performed using SPSS version 26.0 (IBM, USA). A two-sided p < 0.05, with all tests being two-tailed, was considered statistically significant.
Results
Patient enrollment and baseline characteristics
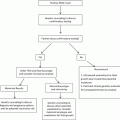
From January 2019 to December 2022, a total of 69 patients with HSIL/CIN2 and HR-HPV were identified initially at the Center for Vulvovaginal Cervical Diseases of the Affiliated Hospital of North Sichuan Medical College. Among them, 34 nulligravid women met the inclusion criteria. However, three patients were lost to follow-up, resulting in 31 patients completing the full study ( Fig. 1 ).

Table 1 summarizes the demographic and clinical characteristics of study participants. The mean age of this study cohort was 26.52 ± 3.67 y, ranging from 19 to 34 y. For HR-HPV genotyping, 17 patients were positive for HPV 16/18, and 14 cases had other subtypes. The TCT results showed 21 cases of NILM, three cases of ASC-US, four cases of LSIL and three cases of HSIL. Colposcopy findings revealed 13 patients with a type 1 transformation zone and 18 cases with a type 2 transformation zone. As for colposcopy grading, 12 patients were classified as minor and 19 as major grade.
| Characteristics | Cases [ n (%)] | |
|---|---|---|
| Age | ≤25 | 10 (32.56) |
| >25 | 21 (67.74) | |
| HPV genotypes | HPV 16/18 | 17 (54.64) |
| Others | 14 (45.16) | |
| Number of HPV subtypes | Multiple | 12 (38.70) |
| Single | 19 (61.29) | |
| TCT results | NILM | 21 (70.0) |
| ASC-US | 3 (9.67) | |
| LSIL | 4 (12.90) | |
| HSIL | 3 (9.67) | |
| Colposcopy findings description | Normal | 0 (0.00) |
| Minor grade | 12 (38.71) | |
| Magor grade | 19 (61.29) | |
| Transform zone type | I | 13 (41.94) |
| II | 18 (58.06) |
HR-HPV clearance and TCT after treatment
Statistical analysis revealed a significant difference in the cumulative HR-HPV clearance rates between 6 and 12 mo post-treatment (35.48% vs. 71.49%, p = 0.005) ( Table 2 ) after treatment. The median time for HR-HPV clearance was 5 mo (95% confidence interval: 5.55–7.77). Furthermore, the remission rate of multiple HPV subtype infections was significantly different compared to single HPV at both 6 mo (8.33% vs. 52.63%, p = 0.034) and 12 mo (53.86% vs. 94.44%, p = 0.026). Although the clearance rate was higher for HPV 16/18 compared to non-HPV 16/18 (81.25% vs. 66.67%), the difference was not statistically significant ( p = 0.605). The 6-mo post-treatment HR-HPV clearance rate was associated with HPV subtype, TCT subtype and colposcopy grade. The 12-mo post-treatment HR-HPV clearance rate was not associated with age, HPV genotype, TCT subtypes or transform zone type ( Table 3 ); however, it was related to multiple coinfection subtypes and colposcopy grade.
| Pathological Response | N (%) | 6 Mo | 12 Mo | ||||
|---|---|---|---|---|---|---|---|
| HPV—Persistent Infection ( n ) | HPV—Negative ( n ) | Clearance Rate | HPV—Persistent Infection ( n ) | HPV—Negative ( n ) | Clearance Rate | ||
| Complete response | 22 (70.96) | 13 | 9 | 40.91 | 2 | 20 | 90.91 a |
| Partial response | 8 (25.80) | 6 | 2 | 25.00 | 5 | 3 | 37.50 |
| Persistence | 1 (3.23) | ༑ | 0 | 0.00 | 1 (3.23) | 0 | 0.00 |
| Progression | 0 (0.00) | / | / | / | 0 (0.00) | / | / |
| Total | 31 (100) | 20 | 11 | 35.48 | 8 | 23 | 71.49 |