Abstract
Palpation has been the standard of care for clinical evaluation of soft tissues until the development of elasticity imaging (EI) in the past two decades. EI is a medical imaging modality that maps elastic properties of soft tissues. EI applications in the breast and liver have become commonplace in clinical practice. Therefore most commercial US systems now have EI software available to image and/or quantify tissue stiffness/elasticity. Obstetric EI is still in very early phases of research focused primarily on the cervix and some research on the uterus and placenta. This chapter describes basic concepts and terminology of EI, provides an overview, and discussion of current research of each EI method in obstetrics.
Keywords
elasticity imaging, cervical stiffness, preterm birth prediction, placental elasticity
Introduction
Palpation, the technique by which clinicians evaluate a patient by touch, has been fundamental to medical practice for thousands of years. Information about tissue stiffness can provide diagnostic information about the presence or status of disease, or about structural or microstructural changes in tissue because of physiologic and pathologic processes. Quantification of this type of information, however, did not emerge until the past two decades, with the advent of elasticity imaging (EI). EI is a medical imaging modality that maps elastic properties of soft tissue. Although B-mode ultrasound (US) imaging remains the standard modality in obstetrics (brightness mode, Chapter 169 ), EI is gradually being introduced. While it has not yet found a place in obstetric clinical practice, it may ultimately complement conventional imaging, if only to improve image contrast in soft tissues because the range of tissue stiffness is much wider than the range of tissue echogenicity that creates B-mode images.
Some EI applications, such as breast and liver, are commonplace in clinical practice. Therefore most high-end clinical systems have some variant of an elasticity/stiffness software package. Obstetric EI is still in its infancy, with research focused primarily on the cervix, somewhat on the uterus and placenta and to a lesser extent, on the fetus. This chapter describes basic concepts and terminology of EI and briefly reviews potential applications in obstetrics.
Elasticity Imaging
Elasticity describes the restoring forces of an object or material to resume its original shape after being subjected to a deforming force (stretching or compressing). It can be quantified if the applied stress (force per unit area) and strain (relative change in shape) are both known. Typical moduli to describe elastic quantities are Young’s modulus (E) and Shear modulus (G). However, imaging modalities must rely on metrics that are related to elastic properties, because the stress distribution in the body is complex and usually unknown, and soft tissues have complicated boundary conditions that require derivation of precise equations specific to type of applied force and tissue type. Therefore understanding the principles of EI is essential to applying this technique to clinical practice. There are three basic approaches to EI: strain (static) elastography (SE), dynamic elastography, and transient elastography. These vary with respect to the type of force applied and the quantity of measure, as described subsequently and in Table 170.1 .
| Method | Description |
|---|---|
| Strain (static) elastography | Qualitative image of relative strain. Compress at skin surface with probe or with physiologic movement and compare before and after compression frames to compute strain. |
| Dynamic elastography: acoustic radiation force imaging | Qualitative image of relative displacement. Produce local displacement using focused US pulse and track displacement. |
| Dynamic elastography: shear-wave elasticity imaging | Quantitative point measure (left) or image (right) of SWS. Shear-wave is produced by ARF pulse and peak displacement is tracked to calculate speed. |
| Transient elastography | Quantitative point measure of SWS. Shear-wave is produced by a mechanical “punch.” Speed is converted to Young’s modulus (currently only applicable to liver.) |
Strain Elastography
SE was developed in the 1970s. For SE, force is applied to the tissue surface through mechanical or manual compression of the US transducer, or by taking advantage of normal physiologic movement, such as arterial pulsations. Comparison of US frames before and after compression allows tracking of tissue displacement and subsequent conversion to strain, typically displayed as an image (elastogram). This image is qualitative rather than quantitative, because strain is a relative measurement of stiffness and its value changes based on amount of pressure applied. Images (and thus interpretation) can be enhanced by performing multiple compression cycles and calculating incremental strain (persistence) because accumulation of multiple frames reduces noise in elastogram images. Displays can show B-mode images side-by-side with a grayscale strain image for direct comparison or overlay a color map on top of the B-mode. A good strain image starts with a good B-mode image and requires adjustments in palpation speed and pressure to display the detail of interest. Many systems have a “quality” metric on the US system screen to inform the operator of necessary adjustments for optimal strain imaging. Such metrics are often mistakenly interpreted as a measure of force applied by the operator with the transducer.
It is unfortunate that the US system cannot provide such information, because it is critical; a small or inconsistent (i.e., nonuniform) stress applied to the target tissue will produce strain with limited penetration and poor homogeneity in stress. Another factor that complicates interpretation of elastograms is that the amount of strain is dependent on depth from the transducer. Further, softer tissue strains more when adjacent to stiffer tissue, and areas of high strain may be seen at boundaries because of the slipping of tissue, which creates artefact.
Attempts to make SE more quantitative include comparing strain between the target tissue and a reference material using strain ratios or quantifying strain heterogeneity. For this approach, it is critically important that the reference tissue be evaluated under the same stress conditions as the target tissue. This technique works well for evaluating tissues such as the breast, where assessing relative strain is useful because tumors typically have different stiffness properties than surrounding normal tissue. The approach does not work as well for the cervix, because the goal is to evaluate overall tissue stiffness.
Strain Elastography in the Cervix
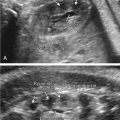
To date, the most developed clinical application of SE in obstetrics is evaluation of the pregnant cervix to determine risk of preterm birth or success of labor induction. Studies first appeared in 2006 but failed to show significant changes in cervical elasticity during pregnancy. Swiatkowska and Preis reported that strain imaging could predict successful induction of labor, but noted that the inability to standardize measurements limited meaningful comparisons between women. Molina et al., in an attempt to reproduce prior results, suggested that SE values may be merely a reflection of the force applied, not a measure of underlying tissue stiffness ( Fig. 170.1 ).
Since then, attempts have been made to quantify the force applied to the cervix. For instance, Hernandez-Andrade et al. studied the ability of SE at 16–24 weeks to predict subsequent preterm birth in a study of 189 women. The sonographer manually applied continuous oscillatory pressure to the cervix with the transducer. Quantification was accomplished by analyzing three cervical projections (cross-sectional at the internal os, cross-sectional at the external os, and sagittal in the midcervix at the level of the cervical length measurement). Strain values represented the percentage of tissue displacement averaged among consecutive frames. Women whose percentage displacement was ≤25th percentile (i.e., less deformation, meaning stiffer tissue) had a lower risk of delivering preterm. Specifically, 20 of 135 (15%) women with strain values >25th percentile delivered preterm compared to one of 51 (2%) women with strain values ≤25th percentile.
There have been several other studies of cervical SE, and a common problem addressed by all of their investigators is that of quantification of force.
Strain Elastography in the Placenta
A handful of studies have used SE to evaluate the placenta. For instance, Cimsit et al. used SE to evaluate the placenta in 119 patients (28 with preeclampsia, 15 with history of preeclampsia, and 101 healthy) and found that those with early onset preeclampsia had significantly higher strain ratios. One issue with SE in the placenta is that it has arguably even greater complexity of boundary conditions than the cervix. Nevertheless, if transducer force could be standardized, SE could have significant promise.
Dynamic Elastography
Acoustic Radiation Force Imaging
Dynamic elastography requires displacing tissue with some type of US pulse. One of these approaches, acoustic radiation force impulse (ARFI) imaging, uses focused, long-duration, high-intensity US pulses that produce tissue displacement up to 10 microns. The degree of displacement is related to tissue stiffness; softer tissues will displace more than stiffer tissues. This method is much less user-dependent than SE, although it must be noted that pressure by the transducer (i.e., prestress, or preload) may itself cause tissue stiffening. Also when evaluating highly attenuating (i.e., US-wave absorbing) tissues, limited penetration is possible because of absorption of the pushing pulse, as well as potential reduction of frame rate caused by limitations on thermal and mechanical indices required to produce high-power pulses, either of which could yield poor results.
Shear-Wave Elasticity Imaging
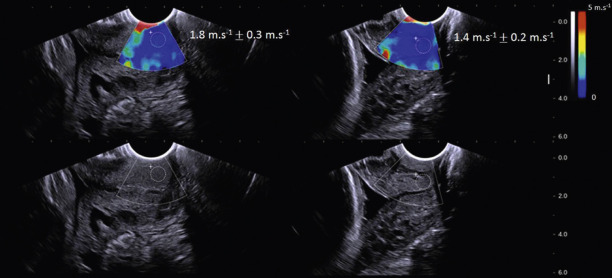
Shear-wave elasticity imaging (SWEI), another form of dynamic elastography, is very similar to ARFI because it uses the same excitation pulse to displace the tissue but instead quantifies the speed of the shear wave produced by the excitation. The displacement produced by the moving wave at several positions radiating from the push can be tracked and used to estimate shear-wave speed (SWS). Shear waves travel faster in stiffer tissue and slower in softer tissues and under simple conditions E and G can be directly calculated. Systems either display a point measure (estimate the speed in a small box, approx. 5×5 mm) or at multiple locations to produce an image (sweep push pulses at multiple locations). SWS point measures have higher precision and accuracy because the wave is tracked over longer distances but do not show spatial variations in speeds/stiffness. SWS images have higher variance at any given location as compared to point measures because the displacement is tracked over shorter distances to show spatial variations. When making SWS measurements, it is important to choose a locally homogeneous region in the tissue away from boundaries and structures that may disrupt wave propagation (e.g., blood vessels). Generating SWSs near boundaries or in thin tissues may introduce bias and variance in results because wave generation is unpredictable near boundaries (including the boundary of the tissue which is in contact with the transducer). It should be noted that, because SWEI techniques are still under active development (more accurate estimation algorithms of SWS), there is not yet a reliable metric to objectively confirm data quality.
Shear-Wave Elasticity Imaging in the Cervix.
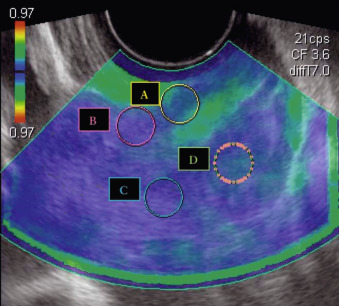
SWEI has been applied to the pregnant cervix. Unfortunately, producing adequate shear waves in the cervix is a complex challenge. As discussed earlier, thin, heterogeneous tissues violate many assumptions needed to quantify elastic moduli. In addition, cervical tissue is highly attenuating (wave energy is rapidly lost) because of its microstructural complexity. These characteristics, particularly its thinness (radius 12–15 mm) cause difficulties. For this reason, a transvaginal transducer may not be ideal because its large acoustic elements limit how close to the transducer reliable shear waves can be produced.
In a cross-sectional study of women at 24–35 weeks’ gestation, Muller et al. found a “slight” decrease in SWS in a group hospitalized for preterm labor ( n = 81, of which 12% delivered preterm) compared to a control group ( n = 27, of which 4.3% delivered preterm). Interestingly, they did not find a significant difference in cervical stiffness between the first and second trimesters, unlike several other investigators who used other (some nonelastography based) methods of cervical stiffness evaluation. The authors mention the high variance in their first-trimester estimates, noting this could explain the nonsignificance of their results. It is imaginable that variability was so high because of the use of a standard transvaginal transducer, as described earlier. Fig. 170.2 shows how close the transducer (tissue boundary) is to the region of interest (white circle). They did find a statistically significant difference between the first and third trimesters.