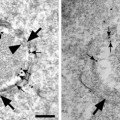
Fig. 1.
(a, b) show various early and late endocytic compartments in human hepatoma cells after 30 min WGA-HRP internalization as visualized by electron dense DAB precipitates that were generated either in vivo prior to HPF (a) or after glutaraldehyde fixation (b). Complex endocytic compartments (long arrows ) frequently consisting of a multivesicular vacuolar part (asterisk in a) and multiple domains with different appendices are localized near the cell surface (a) or close to the Golgi apparatus (b). Extended endocytic trans-Golgi networks are visible in both panels. As can be seen in (a), reactions are lacking at the cell surface because of the treatment with ascorbic acid by which extracellular DAB oxidation is avoided and cells are protected from becoming walled by reaction products. GA Golgi apparatus, TGN trans-Golgi networks, N nucleus.
Since HRP retains its catalytic activity after aldehyde fixation, the DAB oxidation is usually performed after chemical fixation. An improvement of the method was achieved by introducing a protocol for peroxidase-DAB-cytochemistry of living cells at 4°C, based on procedures originally used for cell fractionation (16) and immunocytochemical studies of endosomes by whole-mount electron microscopy (17), and by combining this protocol with high pressure-freezing (HPF) (18). HPF permits both ultrafast immobilization of dynamic cellular processes such as endocytosis and excellent fine structural preservation (for review, ref. (19)). Our combined cytochemical-HPF technique is very suitable for studies of the complex endocytic architectures and 3D-analyses by electron tomography.
2 Materials
2.1 Reagents
1.
II Phosphate buffered saline (PBS); purchased as tablets.
2.
Tris (hydroxymethyl) aminomethane (TRIS; 2-amino-2-(hydroxymethyl)-1,3-propanediol; H2NC(CH2OH)3); 0.05 M Tris–HCl buffer, pH 7.4.
3.
Cacodylic acid sodium salt (sodium cacodylate; dimethylarsinic acid sodium salt trihydrate; (CH3)2AsO2N2 . 3H2O); 0.1 M cacodylate buffer pH 7.4.
4.
Veronal sodium salt (5,5-diethylbarbituric acid sodium salt; C8H11N2NaO3).
5.
Sodium acetate (acetic acid sodium salt; CH3COONa).
6.
HEPES-buffer 1 M (4-(2-hydroxyethyl)-1-piperazinethansulfonic acid).
7.
Glutaraldehyde (pentane-1,5-dial; OHC(CH2)3CHO); electron microscopy grade 25%.
8.
Osmium tetroxide (OsO4); crystalline.
9.
Potassium ferrocyanide (potassium hexacyanoferrate (II) trihydrate; K4(Fe(CN)6)·3H2O).
10.
3,3′-Diaminobenzidine tetrahydrochloride (DAB; (NH2)2C6H3C6H3(NH2)2 . 4HCl).
11.
Graded series of ethanol.
12.
Epon 812 (glycid ether 100; 1,2,3-propanetriol glycidyl ether); epoxy resin of low viscosity.
13.
2-Dodecenylsuccinic acid anhydride (DDSA; C16H26O3); Epon hardener.
14.
Nadic methyl anhydride (NMA; methylnorbornene-2,3-dicarboxylic anhydride); Epon hardener.
15.
2,4,6-Tris(dimethylaminomethyl)phenol (DMP-30); accelerator for epoxy polymerization.
2.2 Media and Solutions
1.
Cell culture medium for HepG2 cells: Minimum Essential Medium with Eagle salts (MEM) containing 10% Fetal Bovine Serum (FBS), 2 mM l-glutamine, and 1% Non-essential Amino Acid Solution (NEAA) (see Note 1).
2.
WGA-HRP solution: First, prepare a stock solution of 100 μg/100 μL WGA-HRP (Sigma) in double distilled water (ddH2O) and ultrasonicate. Then, dilute the stock solution with complete culture medium to a final concentration of 33 μg/100 μL (see Note 2).
3.
Glutaraldehyde fixative: Dilute 25% glutaraldehyde (electron microscopy grade, stored at 4°C) to 2.5% with 0.1 M cacodylate buffer (pH 7.4) (see Note 3).
4.
DAB solution for use after glutaraldehyde fixation of cells: Dissolve DAB (3,3′-diaminobenzidine tetrahydrochloride) in 0.05 M Tris–HCl buffer (pH 7.4) by ultrasonication to obtain a final DAB concentration of 0.5 mg/mL. Afterwards, filter the solution through filter paper (Whatman grade 2). Prepare the DAB solution immediately prior to use and keep in the dark.
5.
HEPES-buffered culture medium: Dilute 1 M HEPES-buffer solution in culture medium to a final molarity of 20 mM. Prepare the buffer immediately prior to the experimental procedure and warm it to 37°C.
6.
DAB solution for in vivo use before HPF of cells: Dissolve 70 mM NaCl and 50 mM l-ascorbic acid in ddH2O. Add 1 M HEPES-buffer to a final concentration of 20 mM. Set the pH to 7.0–7.2. Then, add DAB to a final concentration of 1.5 mg/mL. After ultrasonication and filtering the solution through filter paper (Whatman grade 2), check the pH value again and adjust the osmolarity to 300 mOsmol/kg with crystalline NaCl. Prepare the DAB solution immediately prior to use and keep it at 4°C in the dark (see Note 4).
7.
Hydrogen peroxide solution: Dilute 30% hydrogen peroxide solution (medical extra pure, stabilized) to 1% in ddH2O (see Note 5).
8.
Aqueous osmium tetroxide solution: Dissolve the content of sealed ampulle of osmium (VIII) oxide (OsO4) in ddH2O to a final concentration of 2%. Store the solution at 4°C until use (see Note 6).
9.
Osmium-ferrocyanide solution: Dissolve potassium hexacyanoferrate (II) trihydrate to a final concentration of 3% with ddH2O and store at ambient temperature. Before use, mix it with an equal volume of 2% aqueous osmium tetroxide.
10.
Buffered osmium tetroxide solution: Prepare solution A by dissolving 7.35 g veronal sodium salt and 4.85 g sodium acetate (anhydrous, pro analysis grade) in 250 mL ddH2O. Prepare solution B by dissolving 21.25 g NaCl in 250 mL ddH2O. To prepare a 1% OsO4 solution, mix 20 mL of solution A, 7 mL of solution B, 22 mL of 0.1 M HCl, and 50 mL of 2% aqueous osmium tetroxide. Store the solution at 4°C until use.
11.
Preparation of the epoxy resin for embedding: Mix 131 mL glycid ether 100 (Epon 812), 50 mL DDSA, and 89 mL methylnadic anhydride (MNA) under gentle stirring. Afterwards, add 5.4 mL DPM-30 and mix well under gentle stirring. Let the resin mixture sit for 30 min, aliquot it in small glass bottles, freeze and store it at −20°C.
12.
Uranyl acetate solution: Dissolve uranyl acetate dihydrate to a final concentration of 1% in ddH2O. Store it at room temperature until application.
13.
Lead citrate solution: Dissolve 1.33 g lead (II) nitrate and 1.76 g sodium citrate in 30 mL ddH2O, mix well, and leave it for 30 min before addition of 8 mL 1 M NaOH. Afterwards, add ddH2O to a final volume of 50 mL and ultrasonicate the solution until the salts are completely dissolved. Store the solution at ambient temperature until use.
14.
PBS: Dissolve pre-packed units in an appropriate volume of ddH2O yielding 0.01 M phosphate buffer, 0.0027 M potassium chloride, and 0.137 M sodium chloride. Adjust the pH to 7.4.
15.
OTE-solution: Dissolve OTE powder (Oolong tea extract, purchased from Nisshin EM Co. Ltd. Tokyo, Japan) in PBS pH 7.4 to a final concentration of 0.2%.
2.3 Equipment
An HPF machine such as HPM 010 from BAL-TEC (Principality of Liechtenstein), sapphire discs (3 mm diameter, BAL-TEC), and a cryo substitution machine such as Leica AFS system (Leica Microsystems) are required. Furthermore, general laboratory items such as gloves, work coats and masks, miscellaneous glass and plastic ware, adjustable pipettes, glass filtration device, filter paper, pH test strips, Beem capsules (polyethylene molds), Parafilm, and fine point tweezers for manipulation of the coverslips should be available as well as equipment required for cell culture, resin embedding of the cells, the preparation of ultrathin sections, and an transmission electron microscope.
3 Methods
3.1 General Cell Culture Procedures
Human HepG2 hepatoma cells and other normal and tumor cell lines (all from American Type Culture Collection) were grown under conditions recommended by the supplier. The cell cultures were used at 60–80% confluency for the experiments, usually 48 h after seeding. Cells were grown on different types of surfaces depending on the experimental procedure, i.e., on glass coverslips for DAB oxidation after chemical fixation or on sapphire discs placed onto glass coverslips for in vivo DAB oxidation and HPF (see Fig. 2).