Juvenile nasopharyngeal angiofibromas and paragangliomas are the most common hypervascular tumors of the head and neck that require embolization as an adjunct to surgery. A detailed understanding of the functional vascular anatomy of the external carotid artery is necessary for safe and effective endovascular therapy. Embolization, using a transarterial technique and particulate agents, a direct puncture technique and liquid embolic agents, or both techniques may allow for complete devascularization of hypervascular tumors of the head and neck. Effective embolization of these tumors results in a significant reduction of blood loss during surgery and allows for complete resection of the tumors. Use of meticulous technique and a thorough knowledge of functional anatomy of the head and neck vasculature are essential.
This article provides an overview of embolization of vascular tumors of the head and neck, with emphasis on recent advancements in endovascular techniques available for treatment. The authors first discuss the functional vascular anatomy and technical aspects of head and neck embolization. In the subsequent sections, they present a detailed discussion of the two most common head and neck tumors, juvenile nasopharyngeal angiofibromas (JNAs) and paragangliomas, which require embolization as an adjunct to surgery. Finally, the authors discuss their experiences at the University of Michigan.
Functional vascular anatomy
An understanding of the anatomy of the external carotid artery (ECA) is essential for performing safe and effective embolization of vascular tumors of the head and neck because of the many anatomic variations, territorial anastomoses, and collateral supplies found in this region. The anatomy of the ECA is variable and is best considered on a functional basis. In particular, for cases in which one artery is small, that area is then supplied by an enlarged neighboring branch. The blood supply to tumors of the head and neck is derived from regional vasculature and is provided by branches of the ECA with additional recruitment of the vertebral artery (VA), internal carotid artery (ICA), and thyrocervical or costocervical trunk, depending on the size and location of the tumor. Tumors adjacent to the brain may parasitize regional pial blood supply as they enlarge.
Prevention of serious complications requires knowledge and recognition of the territorial anastomoses. Anastomotic pathways exist between the ECA, ICA, VA, ophthalmic artery, ascending cervical artery, deep cervical artery, and spinal arteries, which are embryologic remnants from early fetal life. The most common dangerous anastomoses involve communications of the first- or second-order branches of the ECA (the ascending pharyngeal [APA], occipital [OA], middle meningeal [MMA], accessory meningeal [AMA], and distal internal maxillary arteries [IMA]) with the ICA or VA. Furthermore, the MMA, IMA, superficial temporal, and facial arteries can all anastomose with the ophthalmic artery. These anastomoses may not be evident on an initial angiogram, but may reveal themselves as the changes in the regional blood flow occur during the embolization. Endovascular surgeons need to be familiar with these connections when embolizing tumors within any of these vascular territories to avoid inadvertent passage of the embolic material to the retina or the central nervous system. The ECA system provides collateral circulation for the ICA and is the primary blood supply to many of the cranial nerves. Palsies of cranial nerves V, VII, IX, X, XI, or XII may result from inappropriate embolization of the feeding branches to the vasa nervosum. Selection of the correct embolic agent and, if required, provocative testing before embolization may help to avoid damage to the cranial nerves.
Technical aspects of head and neck tumor embolization
The tumors that require embolization in the head and neck most commonly include paragangliomas and JNAs. Other tumors that may require preoperative embolization include hypervascular metastases, schwannomas, rhabdomyosarcomas, extracranial meningiomas, esthesioneuroblastomas, neuroblastomas, endolymphatic sac tumors, and hemangiopericytomas.
The goal of tumor embolization is to selectively occlude the ECA feeders using intratumoral deposition of the embolic material. The embolic agents commonly used include the following:
- •
polyvinyl alcohol
- •
trisacryl microspheres ( Figs. 1–4 )
Fig. 1
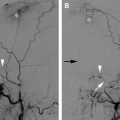
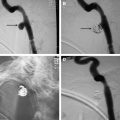
Embolization of large JNA. ( A ) Enhanced CT scan of the skull base reveals a dumbbell-shaped, partially necrotic, and hypervascular mass on the left side. It originates in the region of the sphenopalatine foramen, which is also widened, and extends medially in the nasopharynx and laterally toward the infratemporal fossa. ( B ) Lateral angiogram of the left common carotid artery (CCA) demonstrates a hypervascular mass supplied by the IMA ( single arrow ), branches of the MMA ( double arrows ), and petrous and cavernous branches of the ICA ( arrowheads ). ( C ) Lateral CCA angiogram obtained after particle embolization of the distal IMA and branches of the MMA and APAs that supplied this neoplasm. Small amount of residual blush is present, supplied by tiny branches of the ECA and ICA ( arrows ). These branches are difficult to catheterize and embolize. ( D ) Transnasal ( double arrows ) and temporal approach ( single arrow ) was used for percutaneous access into the tumor bed, and Onyx was injected into the tumor bed using fluoroscopy ( arrowheads ). ( E ) Near-complete devascularization of the tumor is shown on this postembolization CCA angiogram. Note residual, minor opacification of the cavernous branch of the ICA ( arrowhead ).
Fig. 2
Large, left-sided, carotid body paraganglioma (CBP), embolized using intra-arterial particulate material (250 cc total blood loss at resection). ( A ) Lateral multiplanar reformation of a CT angiogram shows a hypervascular mass (M) splaying the carotid bifurcation (C bifur). Left external carotid artery (LECA) is displaced anteriorly. ( B ) Axial, contrast-enhanced CT image of the neck shows a hypervascular mass (M) displacing the left internal carotid artery (LICA) posteriorly and the LECA anteriorly. ( C , D ) Ultrasound image ( C ) demonstrates a predominantly hypoechoic mass, which appears extremely vascular using color Doppler ( D ). ( E ) Lateral common carotid angiogram before embolization demonstrates this hypervascular mass (M) splaying the carotid bifurcation, a large area of blush, and multiple hypertrophied arterial feeders. ( F ) Lateral common carotid angiogram from late in the arterial phase reveals the extent of tumor blush and the complete margins of this hypervascular mass (M). ( G ) Lateral angiogram shows a microcatheter in a hypertrophied branch of the APA ( arrow ) before embolization. ( H ) Lateral, postembolization angiogram demonstrates near-complete devascularization. ( I ) Gross pathology photograph shows circumscribed paraganglioma (3.8 cm) with necrotic, whitish-yellow foci ( arrowheads ) associated with intravascular particles ( asterisks ) and surrounding peripheral viable ( tan ) tumor. ( J ) Viable paraganglioma ( left side ) and necrotic paraganglioma ( asterisk ) associated with a large, thin-walled vessel containing numerous particles (trisacryl microspheres, 100–300 μm, original magnification ×4). The particles are round, eosinophilic, and associated with fibrin and inflammatory cells (inset, original magnification ×20). ECA, external carotid; ICA, internal carotid artery; M, mass.
Fig. 3
Embolization of a large CBP using particulate material and percutaneous injection of n-butyl cyanoacrylate (n-BCA) (loss of <50 cc of blood at resection). Given the larger size and more rapid growth of the left CBP, its resection was planned first. ( A ) Axial, contrast-enhanced CT image of the neck shows bilateral hypervascular masses (M) consistent with CBPs at the carotid bifurcation. ( B ) Volume-rendered, three-dimensional reformat again demonstrates the masses at the carotid bifurcation bilaterally. ( C ) 111 indium-octreotide scan reveals increased radiotracer uptake at the carotid bifurcation bilaterally (R bifur, L bifur) and within the mediastinum (Med). ( D , E ) Frontal ( D ) and lateral ( E ) left common carotid angiograms demonstrate at least three hypervascular masses (M) in the region of the carotid bifurcation (C bifur). ( F ) Lateral, external carotid angiogram demonstrates filling of a hypervascular mass (M) from the occipital artery (OC) and a hypertrophied odontoid arcade (OD) off the ascending pharyngeal artery (APA). ( G ) Contrast injection through a needle (N) placed within the hypervascular mass (M) demonstrates filling of the internal tumor architecture. n-BCA glue cast (G) within the tumor from an injection of glue using a different needle (tumorgram, direct injection of contrast into the tumor). ( H , I ) Postembolization frontal ( H ) and lateral ( I ) left common carotid angiograms demonstrate complete devascularization of the mass after injection of from 100- to 300-μm particles and n-BCA (G). ( J ) Photomicrograph of a viable paraganglioma associated with large, thin-walled vessels ( white areas ) containing n-BCA and smaller vessels containing particles (original magnification ×4). The glue has mostly been dissolved out using tissue processing, but thin strands of fibrin remain, outlining where the n-BCA once was (inset, original magnification ×20). M, mass; N, needle.
Fig. 4
Embolization of a large vagal paraganglioma (VP) using particulate material and percutaneous injection of Onyx (loss of <50 cc of blood at resection). ( A ) Axial, fat-saturated, T2-weighted MR image demonstrates a large mass within the neck (M). Note several flow voids within the tumor. RCCA, right common carotid artery. ( B ) Axial CT scan of the skull base with bone windows demonstrates a normal jugular bulb ( J ). ( C , D ) Frontal ( C ) and lateral ( D ) right subclavian arteriograms demonstrate a large hypervascular mass (M) with supply off the deep cervical (DC), superficial cervical (SC), and right vertebral arteries (RVA). ( E ) Lateral right common carotid arteriogram revealing an extremely vascular mass (M) located posteriorly in the carotid sheath. It displaces the right external carotid artery (RECA) and right internal carotid artery (RICA) anteriorly. ( F ) Lateral selective microcatheter angiogram of the occipital artery before particle embolization displays filling of the tumor vessels in this mass (M). ( G , H ) Lateral right subclavian arteriogram ( G ) and common carotid angiogram ( H ) after particulate and coil embolization of the arterial feeders from the right external carotid artery using trisacryl microspheres from 100 to 300 μm demonstrates residual filling of the mass (M). ( I ) Frontal contrast injection through the needle into the tumor, tumor gram (N) demonstrates opacification of the internal vascular architecture of the mass (M). ( J ) Frontal spot radiograph demonstrates the Onyx (O) cast with the mass. ( K ) Frontal right subclavian arteriogram demonstrates near-complete devascularization of the hypervascular mass. ( L ). Lateral right common carotid arteriogram confirms near-complete devascularization. ( M ) Photomicrograph of the resected specimen reveals a viable paraganglioma associated with large, thin-walled vessels containing numerous particles (trisacryl microspheres) and Onyx (black) (original magnification ×4). The Onyx is composed of black granular material that is nonrefractile and is associated with fibrin and inflammatory cells (Inset, original magnification ×20). C, coils; N, needle.
- •
liquid n-butyl cyanoacrylate (n-BCA) Trufill (Cordis Neurovascular Inc., Miami Lakes, Florida) (see Fig. 3 )
- •
ethyl-vinyl alcohol copolymer (EVOH) Onyx (ev3, Irvine, California) (see Figs. 1 and 4 )
- •
gelfoam pledgets
- •
microcoils.
The embolization is ideally performed from 24 to 72 hours before the surgical resection to allow time for maximal thrombosis of the occluded vessels and prevent recanalization of the occluded arteries or formation of collateral arterial channels. Preoperative embolization is cost-effective and tends to shorten operative time by reducing blood loss and the period of recovery.
Treatment begins with first obtaining a detailed cerebral angiogram that includes selective injections of the common carotid artery, ICA, ECA, VA, and thyrocervical and costocervical trunks of the subclavian artery. A microcatheter is then advanced using fluoroscopic guidance into the artery supplying the tumor, and a microcatheter angiogram is performed to check for dangerous anastomoses between the ECA and ICA or vertebral arteries. The appropriate embolic agent is then injected using constant fluoroscopic monitoring, making sure to avoid reflux of embolic material and being vigilant for any dangerous anastomoses. If critical anastomoses are present, the anastomotic connection can be occluded using coils and then the particulate embolization can be performed. Ideally, the embolic material is deposited at the arteriolar/capillary level. If there is arteriovenous shunting within the tumor, the particle size may need to be increased to prevent passage into the venous side. Proximal occlusion of the arterial feeders is inadequate because it allows arterial collateralization and may make surgical removal more difficult.
The authors prefer using trisacryl microspheres of 100 to 300 μm because these particles allow more distal penetration into the tumor bed and better devascularization. However, one should always be aware of the possible risk for devascularizing the cranial nerves (the vasa nervosum are usually smaller than 60 μm) and the skin. In addition to potential central and peripheral nervous system damage, undesired embolization of normal external carotid territories can cause mucosal and tongue necrosis, laryngeal damage, and ocular damage. Smaller particles may also increase the risk for tumoral hemorrhage and swelling. When embolizing the arterial pedicles that might also supply the cranial nerves (eg, the stylomastoid branch of the OA or the neuromeningeal trunk of the APA), the authors increase the particle size to from 300 to 500 μm. Similarly, the authors generally avoid liquid embolic agents (eg, n-BCA or EVOH), preferring to use a transarterial approach with particulate material, because liquid embolic agents can potentially occlude the arterial supply to the cranial nerves and may pass through the tiny anastomoses into the intracranial circulation.
Direct percutaneous puncture of tumors when using fluoroscopic, ultrasound, or CT guidance has also been described as a method to embolize a number of different tumors (see Figs. 1, and 4, 3 ). The method was initially reported for use in tumors in which conventional transarterial embolization was technically impossible because of the small size of the arterial feeders or involvement of branches arising from the ICA or VA feeding the tumor. Examples include large tumors with supply from the ICA, VA, or ophthalmic artery for which devascularization from an intra-arterial approach using a microcatheter may not be possible or for which there may be significant risk for reflux of particles into the intracranial circulation or the retina. Excellent results obtained using this technique have extended its application to smaller and less complex tumors. Direct and easy access to the vascular tumor bed that is not hampered by arterial tortuosity, the small size of the arterial feeders, atherosclerotic disease, or catheter-induced vasospasm is the main advantage of this technique.
Complete devascularization of the tumor can be obtained with decreased risk to the patient by using direct tumoral injection of n-BCA or Onyx. Onyx is a liquid embolic agent for presurgical embolization of cerebral arteriovenous malformations that has recently been approved by the US Food and Drug Administration. Onyx is a nonadhesive liquid embolic agent that is supplied in ready-to-use vials in a mixture with EVOH, dimethyl sulfoxide solvent (DMSO) and tantalum. Currently 6% (Onyx 18) and 8% (Onyx 34) EVOH concentrations (dissolved in DMSO) are available in the United States. Onyx is mechanically occlusive but nonadherent to the vessel wall. Its nonadherent properties allow for a slow single injection of the embolic agent over a long period of time. During direct injection, if unfavorable filling of the normal vascular structures occurs, the injection can be stopped and resumed after 30 seconds to 2 minutes. Solidification will occur in the embolized portion of the tumor. The injection can then be restarted, with Onyx taking the path of least resistance and filling another portion of the tumor. As the result of its properties, Onyx may potentially allow for a more controlled injection with better penetration into the tumor bed compared with n-BCA (see Figs. 3 and 4 ). Another benefit is that it advances in a single column, thus reducing the risk for involuntary venous migration.
The authors perform percutaneous injection of n-BCA or Onyx by placing an 18-gauge, short guiding needle into the tumor using fluoroscopic, ultrasound, or CT guidance and then coaxially introducing a 20-gauge spinal needle. After the needle is correctly located within the vascular bed of the tumor, a constant reflux of blood is observed. Contrast agent is injected through the needle and a tumorgram is obtained to assess for arterial reflux, venous drainage, potential for extravasation, and to determine which vascular compartment of the tumor will be filled with n-BCA or Onyx. The injection of the embolic agent is then performed using negative roadmapping. The procedure is stopped after complete devascularization is achieved, as determined by nonvisualization of intratumoral flow, or if the risk for potential arterial reflux into the intracranial circulation is considered to be high.
Technical aspects of head and neck tumor embolization
The tumors that require embolization in the head and neck most commonly include paragangliomas and JNAs. Other tumors that may require preoperative embolization include hypervascular metastases, schwannomas, rhabdomyosarcomas, extracranial meningiomas, esthesioneuroblastomas, neuroblastomas, endolymphatic sac tumors, and hemangiopericytomas.
The goal of tumor embolization is to selectively occlude the ECA feeders using intratumoral deposition of the embolic material. The embolic agents commonly used include the following:
- •
polyvinyl alcohol
- •
trisacryl microspheres ( Figs. 1–4 )
Fig. 1
Embolization of large JNA. ( A ) Enhanced CT scan of the skull base reveals a dumbbell-shaped, partially necrotic, and hypervascular mass on the left side. It originates in the region of the sphenopalatine foramen, which is also widened, and extends medially in the nasopharynx and laterally toward the infratemporal fossa. ( B ) Lateral angiogram of the left common carotid artery (CCA) demonstrates a hypervascular mass supplied by the IMA ( single arrow ), branches of the MMA ( double arrows ), and petrous and cavernous branches of the ICA ( arrowheads ). ( C ) Lateral CCA angiogram obtained after particle embolization of the distal IMA and branches of the MMA and APAs that supplied this neoplasm. Small amount of residual blush is present, supplied by tiny branches of the ECA and ICA ( arrows ). These branches are difficult to catheterize and embolize. ( D ) Transnasal ( double arrows ) and temporal approach ( single arrow ) was used for percutaneous access into the tumor bed, and Onyx was injected into the tumor bed using fluoroscopy ( arrowheads ). ( E ) Near-complete devascularization of the tumor is shown on this postembolization CCA angiogram. Note residual, minor opacification of the cavernous branch of the ICA ( arrowhead ).
Fig. 2
Large, left-sided, carotid body paraganglioma (CBP), embolized using intra-arterial particulate material (250 cc total blood loss at resection). ( A ) Lateral multiplanar reformation of a CT angiogram shows a hypervascular mass (M) splaying the carotid bifurcation (C bifur). Left external carotid artery (LECA) is displaced anteriorly. ( B ) Axial, contrast-enhanced CT image of the neck shows a hypervascular mass (M) displacing the left internal carotid artery (LICA) posteriorly and the LECA anteriorly. ( C , D ) Ultrasound image ( C ) demonstrates a predominantly hypoechoic mass, which appears extremely vascular using color Doppler ( D ). ( E ) Lateral common carotid angiogram before embolization demonstrates this hypervascular mass (M) splaying the carotid bifurcation, a large area of blush, and multiple hypertrophied arterial feeders. ( F ) Lateral common carotid angiogram from late in the arterial phase reveals the extent of tumor blush and the complete margins of this hypervascular mass (M). ( G ) Lateral angiogram shows a microcatheter in a hypertrophied branch of the APA ( arrow ) before embolization. ( H ) Lateral, postembolization angiogram demonstrates near-complete devascularization. ( I ) Gross pathology photograph shows circumscribed paraganglioma (3.8 cm) with necrotic, whitish-yellow foci ( arrowheads ) associated with intravascular particles ( asterisks ) and surrounding peripheral viable ( tan ) tumor. ( J ) Viable paraganglioma ( left side ) and necrotic paraganglioma ( asterisk ) associated with a large, thin-walled vessel containing numerous particles (trisacryl microspheres, 100–300 μm, original magnification ×4). The particles are round, eosinophilic, and associated with fibrin and inflammatory cells (inset, original magnification ×20). ECA, external carotid; ICA, internal carotid artery; M, mass.
Fig. 3
Embolization of a large CBP using particulate material and percutaneous injection of n-butyl cyanoacrylate (n-BCA) (loss of <50 cc of blood at resection). Given the larger size and more rapid growth of the left CBP, its resection was planned first. ( A ) Axial, contrast-enhanced CT image of the neck shows bilateral hypervascular masses (M) consistent with CBPs at the carotid bifurcation. ( B ) Volume-rendered, three-dimensional reformat again demonstrates the masses at the carotid bifurcation bilaterally. ( C ) 111 indium-octreotide scan reveals increased radiotracer uptake at the carotid bifurcation bilaterally (R bifur, L bifur) and within the mediastinum (Med). ( D , E ) Frontal ( D ) and lateral ( E ) left common carotid angiograms demonstrate at least three hypervascular masses (M) in the region of the carotid bifurcation (C bifur). ( F ) Lateral, external carotid angiogram demonstrates filling of a hypervascular mass (M) from the occipital artery (OC) and a hypertrophied odontoid arcade (OD) off the ascending pharyngeal artery (APA). ( G ) Contrast injection through a needle (N) placed within the hypervascular mass (M) demonstrates filling of the internal tumor architecture. n-BCA glue cast (G) within the tumor from an injection of glue using a different needle (tumorgram, direct injection of contrast into the tumor). ( H , I ) Postembolization frontal ( H ) and lateral ( I ) left common carotid angiograms demonstrate complete devascularization of the mass after injection of from 100- to 300-μm particles and n-BCA (G). ( J ) Photomicrograph of a viable paraganglioma associated with large, thin-walled vessels ( white areas ) containing n-BCA and smaller vessels containing particles (original magnification ×4). The glue has mostly been dissolved out using tissue processing, but thin strands of fibrin remain, outlining where the n-BCA once was (inset, original magnification ×20). M, mass; N, needle.
Fig. 4
Embolization of a large vagal paraganglioma (VP) using particulate material and percutaneous injection of Onyx (loss of <50 cc of blood at resection). ( A ) Axial, fat-saturated, T2-weighted MR image demonstrates a large mass within the neck (M). Note several flow voids within the tumor. RCCA, right common carotid artery. ( B ) Axial CT scan of the skull base with bone windows demonstrates a normal jugular bulb ( J ). ( C , D ) Frontal ( C ) and lateral ( D ) right subclavian arteriograms demonstrate a large hypervascular mass (M) with supply off the deep cervical (DC), superficial cervical (SC), and right vertebral arteries (RVA). ( E ) Lateral right common carotid arteriogram revealing an extremely vascular mass (M) located posteriorly in the carotid sheath. It displaces the right external carotid artery (RECA) and right internal carotid artery (RICA) anteriorly. ( F ) Lateral selective microcatheter angiogram of the occipital artery before particle embolization displays filling of the tumor vessels in this mass (M). ( G , H ) Lateral right subclavian arteriogram ( G ) and common carotid angiogram ( H ) after particulate and coil embolization of the arterial feeders from the right external carotid artery using trisacryl microspheres from 100 to 300 μm demonstrates residual filling of the mass (M). ( I ) Frontal contrast injection through the needle into the tumor, tumor gram (N) demonstrates opacification of the internal vascular architecture of the mass (M). ( J ) Frontal spot radiograph demonstrates the Onyx (O) cast with the mass. ( K ) Frontal right subclavian arteriogram demonstrates near-complete devascularization of the hypervascular mass. ( L ). Lateral right common carotid arteriogram confirms near-complete devascularization. ( M ) Photomicrograph of the resected specimen reveals a viable paraganglioma associated with large, thin-walled vessels containing numerous particles (trisacryl microspheres) and Onyx (black) (original magnification ×4). The Onyx is composed of black granular material that is nonrefractile and is associated with fibrin and inflammatory cells (Inset, original magnification ×20). C, coils; N, needle.
- •
liquid n-butyl cyanoacrylate (n-BCA) Trufill (Cordis Neurovascular Inc., Miami Lakes, Florida) (see Fig. 3 )
- •
ethyl-vinyl alcohol copolymer (EVOH) Onyx (ev3, Irvine, California) (see Figs. 1 and 4 )
- •
gelfoam pledgets
- •
microcoils.
The embolization is ideally performed from 24 to 72 hours before the surgical resection to allow time for maximal thrombosis of the occluded vessels and prevent recanalization of the occluded arteries or formation of collateral arterial channels. Preoperative embolization is cost-effective and tends to shorten operative time by reducing blood loss and the period of recovery.
Treatment begins with first obtaining a detailed cerebral angiogram that includes selective injections of the common carotid artery, ICA, ECA, VA, and thyrocervical and costocervical trunks of the subclavian artery. A microcatheter is then advanced using fluoroscopic guidance into the artery supplying the tumor, and a microcatheter angiogram is performed to check for dangerous anastomoses between the ECA and ICA or vertebral arteries. The appropriate embolic agent is then injected using constant fluoroscopic monitoring, making sure to avoid reflux of embolic material and being vigilant for any dangerous anastomoses. If critical anastomoses are present, the anastomotic connection can be occluded using coils and then the particulate embolization can be performed. Ideally, the embolic material is deposited at the arteriolar/capillary level. If there is arteriovenous shunting within the tumor, the particle size may need to be increased to prevent passage into the venous side. Proximal occlusion of the arterial feeders is inadequate because it allows arterial collateralization and may make surgical removal more difficult.
The authors prefer using trisacryl microspheres of 100 to 300 μm because these particles allow more distal penetration into the tumor bed and better devascularization. However, one should always be aware of the possible risk for devascularizing the cranial nerves (the vasa nervosum are usually smaller than 60 μm) and the skin. In addition to potential central and peripheral nervous system damage, undesired embolization of normal external carotid territories can cause mucosal and tongue necrosis, laryngeal damage, and ocular damage. Smaller particles may also increase the risk for tumoral hemorrhage and swelling. When embolizing the arterial pedicles that might also supply the cranial nerves (eg, the stylomastoid branch of the OA or the neuromeningeal trunk of the APA), the authors increase the particle size to from 300 to 500 μm. Similarly, the authors generally avoid liquid embolic agents (eg, n-BCA or EVOH), preferring to use a transarterial approach with particulate material, because liquid embolic agents can potentially occlude the arterial supply to the cranial nerves and may pass through the tiny anastomoses into the intracranial circulation.
Direct percutaneous puncture of tumors when using fluoroscopic, ultrasound, or CT guidance has also been described as a method to embolize a number of different tumors (see Figs. 1, and 4, 3 ). The method was initially reported for use in tumors in which conventional transarterial embolization was technically impossible because of the small size of the arterial feeders or involvement of branches arising from the ICA or VA feeding the tumor. Examples include large tumors with supply from the ICA, VA, or ophthalmic artery for which devascularization from an intra-arterial approach using a microcatheter may not be possible or for which there may be significant risk for reflux of particles into the intracranial circulation or the retina. Excellent results obtained using this technique have extended its application to smaller and less complex tumors. Direct and easy access to the vascular tumor bed that is not hampered by arterial tortuosity, the small size of the arterial feeders, atherosclerotic disease, or catheter-induced vasospasm is the main advantage of this technique.
Complete devascularization of the tumor can be obtained with decreased risk to the patient by using direct tumoral injection of n-BCA or Onyx. Onyx is a liquid embolic agent for presurgical embolization of cerebral arteriovenous malformations that has recently been approved by the US Food and Drug Administration. Onyx is a nonadhesive liquid embolic agent that is supplied in ready-to-use vials in a mixture with EVOH, dimethyl sulfoxide solvent (DMSO) and tantalum. Currently 6% (Onyx 18) and 8% (Onyx 34) EVOH concentrations (dissolved in DMSO) are available in the United States. Onyx is mechanically occlusive but nonadherent to the vessel wall. Its nonadherent properties allow for a slow single injection of the embolic agent over a long period of time. During direct injection, if unfavorable filling of the normal vascular structures occurs, the injection can be stopped and resumed after 30 seconds to 2 minutes. Solidification will occur in the embolized portion of the tumor. The injection can then be restarted, with Onyx taking the path of least resistance and filling another portion of the tumor. As the result of its properties, Onyx may potentially allow for a more controlled injection with better penetration into the tumor bed compared with n-BCA (see Figs. 3 and 4 ). Another benefit is that it advances in a single column, thus reducing the risk for involuntary venous migration.
The authors perform percutaneous injection of n-BCA or Onyx by placing an 18-gauge, short guiding needle into the tumor using fluoroscopic, ultrasound, or CT guidance and then coaxially introducing a 20-gauge spinal needle. After the needle is correctly located within the vascular bed of the tumor, a constant reflux of blood is observed. Contrast agent is injected through the needle and a tumorgram is obtained to assess for arterial reflux, venous drainage, potential for extravasation, and to determine which vascular compartment of the tumor will be filled with n-BCA or Onyx. The injection of the embolic agent is then performed using negative roadmapping. The procedure is stopped after complete devascularization is achieved, as determined by nonvisualization of intratumoral flow, or if the risk for potential arterial reflux into the intracranial circulation is considered to be high.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree