Endoscopic Image-Guided Surgery
Learning neuroendoscopy begins with familiarizing oneself with the equipment. Early frustration often results from lack of understanding of the various neuroendoscopic components. A complete system includes the endoscope, light source, camera, image-recording device, monitor, and transendoscopic instruments. Although the specifics regarding the various equipment options are beyond the scope of this chapter, the authors cannot overemphasize the importance of becoming so acquainted with the various components and that working with the setup become second nature.
This chapter focuses on the cranial disorders in which endoscopy plays a significant role. Some of the techniques discussed here require significant experience before one can perform them safely, whereas other uses for the endoscope can be more broadly applied using basic skills.
 Hydrocephalus
Hydrocephalus
Shunt placement
Although ventricular shunting is a common procedure, a fair percentage of catheters are suboptimally placed. Poor placement may result in poor cerebrospinal fluid (CSF) outflow necessitating repeated transparenchymal passes or may contribute to early shunt failure. Theodosopoulos et al1 have shown that endoscopic ventricular catheter placement significantly increases the percentage of optimally placed catheters over the conventional technique (100% vs 53%, P < 0.001).
In brief, the patient is positioned with the head turned to the opposite side and a shoulder roll placed ipsilaterally. The entry site is prepared in the standard fashion. A ventricular catheter with an opening at the tip is loaded onto the endoscope. The appropriate trajectory is chosen and the catheter is passed into the ventricle. Once the position of the catheter tip is confirmed, the endoscope is removed and the shunt procedure is completed. One must keep in mind that, because the catheter is free to move within the ventricle, the ultimate position of its tip is determined by the entry point and the trajectory. Consequently, endoscopic “guidance” of the catheter tip must be minimized.
Multiloculated hydrocephalus
Multiloculated hydrocephalus results from fibrous septations formed in response to infection or hemorrhage. Such patients may require multiple shunts to adequately drain the CSF spaces. Reducing the patient’s dependency to one catheter minimizes the failure rate and long-term morbidity.
Workup of these patients should include a computed tomographic (CT) ventriculogram to identify the communications between the loculations. Magnetic resonance imaging (MRI) may demonstrate the septations, but it would not provide information regarding the CSF flow dynamics. If the studies demonstrate adequate communication of most of the loculi with distention of just one, it is reasonable to fenestrate the appropriate cyst and attempt to leave the patient shunt free. Approximately 30% of patients with loculated hydrocephalus will not require extracranial CSF diversion following a fenestration procedure.2
Although fenestration of the loculations may be accomplished via a transcortical or transcallosal craniotomy, endoscopy offers a significantly less invasive option. The patient is positioned for either a frontal or occipital approach depending on the location of the loculations. The endoscope is inserted through a burr hole into the ventricular system. Ideally, the scope should first enter a normal ventricle to allow for identification of normal structures. A thin-walled, avascular site is chosen for fenestration. Septal perforation can be performed bluntly with the transendoscopic forceps or with a laser. The communication is enlarged to approximately a 1 cm diameter with the use of a balloon catheter pulled retrograde through the perforation. Bleeding can usually be controlled with irrigation, although occasionally cautery may be needed. Shunting can then be limited to a single intraventricular catheter positioned in the frontal horn.
Lewis and Keiper3 reported a series of 34 patients with uni- or multiloculated hydrocephalus who underwent endoscopic fenestration using a steerable fiberscope and laser fiber. During a mean 26-month follow-up period, cyst fenestration reduced the shunt revision rate from 3.04 per year to 0.25 per year. Patients with multiloculated hydrocephalus were at increased risk for shunt malfunction and cyst recurrence versus patients with uniloculated hydrocephalus. Furthermore, patients who underwent a shunting procedure prior to endoscopy were more likely to require a repeat endoscopic procedure. Compared with craniotomy, endoscopy offers a less invasive approach to treating patients with loculated hydrocephalus, simplifying their shunt system and reducing the rate of shunt failure.
Third ventriculostomy
Despite continued advancements in shunting hardware, extracranial CSF diversion is associated with a significant infection and failure rate that increases with each procedure. Performing a third vetriculostomy is a minimally invasive way of “internally” shunting patients with occlusive hydrocephalus and obviating the need for hardware placement.
Patients with radiological studies consistent with obstructive hydrocephalus are the ideal candidates. However, patients with a combined communicating and non-communicating picture may also benefit. The arachnoid villi are capable of increasing their absorptive capacity in response to an increase in CSF load. CSF isotope clearance studies have been used to predict the absorptive capability of the arachnoid granulations but suffer from a significant false-negative rate.2 In light of the benefits of third ventriculostomy and its low complication rate, we advocate performing the procedure in patients with an obstructive component to their hydrocephalus.
After induction of general anesthesia, the patient’s head is placed in the vertical position on a doughnut. A frontal burr hole is placed 1 cm anterior to the coronal suture and 3 cm to the right of midline. The endoscope is passed transparenchymally into the lateral ventricle (Fig. 10–1). The third ventricle is entered through the foramen of Monro by following the choroid plexus and thalamostriate vein. A blunt forceps is used to perforate the tuber cinerum at the third ventricular floor between the infundibular recess and the mammillary bodies. The pulsation of the basilar artery can usually be identified through the transparent floor and is thus avoided. The perforation is then enlarged with a balloon catheter to maintain patency. The membrane of Liliequist must then be fenestrated to create a communication between the supratentorial and infratentorial cisterns.
Ventricular size does not necessarily correlate with clinical improvement.4,5 Although an MR flow study may demonstrate a flow void at the floor of the third ventricle,5 we elect to follow patients clinically. Patients who fail to improve despite patency of the third ventriculostomy undergo a standard shunting procedure.
Hopf et al6 reported a series of 100 patients who underwent endoscopic third ventriculostomies. Of the 100 procedures, 98 were completed. The overall clinical success rate in the series, which included multiple etiologies, was 76%. The highest success rate was seen with benign space-occupying lesions, although resection of the lesion may have contributed to resolution of the hydrocephalus. Notably, 63% of patients whose etiology for the hydrocephalus was intraventricular hemorrhage responded to third ventriculostomy. The complication rate in this series was 6% with no mortalities. Others, however, report a lower long-term success rate7 and the potential for devastating complications must be kept in mind.5
Third ventriculostomy also may be an alternative to shunt revision in the management of shunt failure in patients with obstructive hydrocephalus. Up to 76.7% of patients may become shunt independent.8 Any preexisting shunt catheter should be removed or ligated to maximize CSF flow through the stoma and maintain its patency. Failures are usually manifest within days and a shunt revision is then performed.
 Colloid Cysts
Colloid Cysts
Colloid cysts are benign lesions that usually arise from the roof of the third ventricle near the foramen of Monro. They can be found, however, throughout the third ventricle, or they may involve the septum pellucidum or fornices.9–12 They contain a gelatinous center of variable viscosity. Although some patients present incidentally, most complain of headaches, nausea, vomiting, memory loss, personality changes, difficulty with gait, or visual deterioration.13
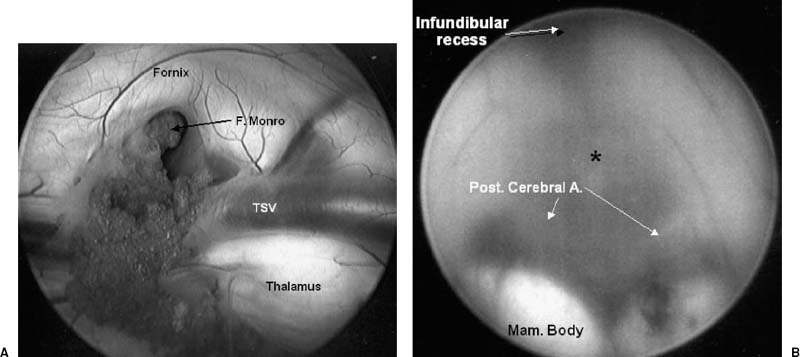
FIGURE 10–1. (A). Endoscopic view within the right lateral ventricle. The choroid plexus can be followed to the foramen of Monro. The thalamostriate vein (TSV) can also be identified. (B). Visualization of the floor of the third ventricle demonstrates the optimal site for performing an endoscopic third ventriculostomy (asterisk). The posterior cerebral arteries (arrows) and the mammillary bodies should be positively identified before penetrating the floor.
The treatment of asymptomatic patients remains controversial. Patients managed by conservative observation must be followed with frequent imaging and should be informed of the early warning signs of obstructive hydrocephalus. We recommend against such a course in patients with lesions greater than 1 cm and those with hydrocephalus. Symptomatic patients clearly require surgical intervention.
Surgical options include transfrontal or transcallosal craniotomy, stereotactic aspiration, ventricular shunting, or endoscopic resection.14 Ventricular shunting is reserved for patients not medically fit to undergo a more definitive procedure. Minimally invasive techniques have been developed to reduce the operative morbidity associated with craniotomy. Stereotactic aspiration is initially effective, but the high recurrence rate has led to this procedure falling out of favor.15
Endoscopic resection of colloid cyst is a minimally invasive method of effecting a definitive treatment. Patients are positioned supine with the head secured in three-point fixation and flexed 15 degrees. A burr hole is placed 1 cm anterior to coronal suture and 4 cm off midline. The right side is chosen unless the left lateral ventricle is unilaterally dilated or the lesion is grossly asymmetrically deviated toward the left. An outer cannula is passed into the lateral ventricle and secured to the operating room table. The zero-degree endoscope is used to determine the relationship of the cyst to the foramen of Monro, choroid plexus, fornices, septum pellucidum, and third ventricle (Fig. 10–2). Small cysts can occasionally be grasped and removed entirely as a single specimen. Excessive traction, however, must be avoided to prevent vascular injury. Larger lesions are punctured and the cyst contents are evacuated via aspiration or intracystic irrigation. If the cyst wall does not separate easily, it is coagulated with bipolar cautery or neodymium:yttrium-aluminum-garnet laser and removed piecemeal using scissors and biopsy forceps. Gross total removal can be achieved in 85% of patients with a > 95% resection in the remainder where complete resection would unnecessarily risk vascular injury.13 We routinely fenestrate the septum pellucidum with a grasping instrument and dilate the opening with a 3F Fogarty balloon catheter. Unlike others,16 we do not leave a ventricular catheter in place. Patients are monitored overnight in the neurosurgical intensive care unit and transferred to the ward the following morning.
We believe that endoscopic resection should be considered the treatment of choice for patients with colloid cysts. Operative time is significantly reduced when compared with craniotomy. In addition, duration of hospital stay is reduced as is the time before returning to work.14 In our series, there were no mortalities or permanent morbidities.13 Furthermore, with a mean follow-up greater than 4 years, there have been no recurrences even in patients with known residual cyst wall.
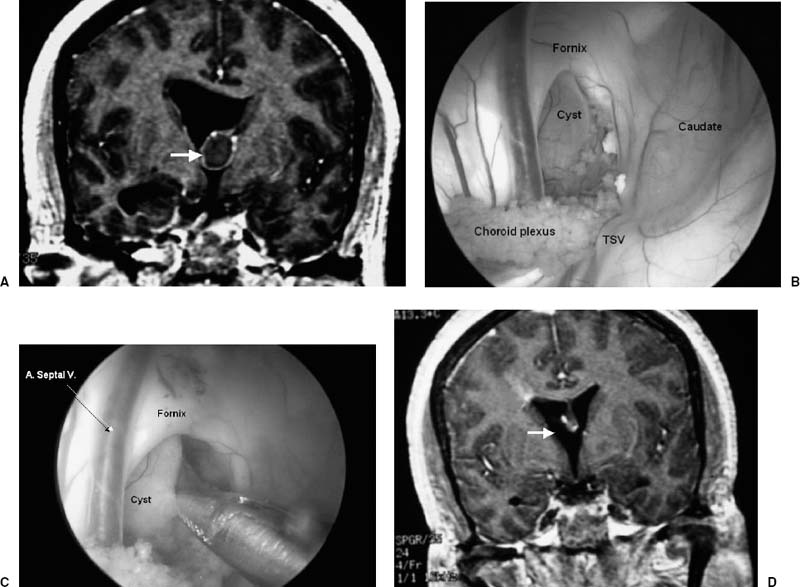
FIGURE 10–2. (A). Coronal magnetic resonance imaging (MRI) demonstrates a colloid cyst at the foramen of Monro (arrow). (B,C). Endoscopic exposure through the right lateral ventricle demonstrates the cyst, which can be excised in a piecemeal fashion. Care must be taken not to injure the thalamostriate vein (TSV) and anterior septal vein (A. Septal V.). (D). Postoperative MRI shows complete tumor removal (arrow).
 Arachnoid Cysts
Arachnoid Cysts
Arachnoid cysts are intra-arachnoid CSF collections usually of congenital origin. They are often found incidentally, although they can produce mass effect and produce symptoms through neural compression.17 Symptomatic cyst should be surgically treated barring medical contraindications. Although many surgical options have been described including stereotactic aspiration and cyst excision,18 the more accepted treatments involve either shunting the cyst to the peritoneum or creating a communication between the cyst and another CSF compartment.
Prior to the advent of endoscopic surgery, shunting the cyst to the peritoneum offered a minimally invasive therapeutic option with a low morbidity. Fenestration required a craniotomy with its increased risk. However, with endoscopic techniques, the arachnoid cyst could be fenestrated with minimal morbidity thereby avoiding the need for shunt catheter placement. Although peritoneal shunting is effective, the shunt failure rate is as high as 10% and shunt dependency may occur.19
Middle fossa arachnoid cysts
The patient is placed in the supine position with the head turned to the contralateral side. A temporal burr hole is placed above the zygomatic arch. Care is taken when introducing the trocar and endoscope not to allow excessive egress of CSF because that would lead to cyst collapse and poor visualization. For orientation, one follows the Sylvian veins or middle cerebral artery toward the basal cisterns. A blunt forceps is used to puncture the arachnoid membrane between the frontal and temporal lobes at the most proximal extent of the Sylvian fissure. A Fogarty catheter is inserted into the cistocisternostomy to enlarge the fenestration. Partial resection of the cyst wall and arachnoid membrane may further prevent delayed occlusion and cyst recurrence. Some authors17 advocate insertion of a fimbrial ventricular catheter to maintain patency.
Suprasellar arachnoid cysts
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree