Carotid cavernous fistulas (CCFs) are abnormal communications between the carotid arterial system and the cavernous sinus. CCFs are broadly classified as either direct or indirect. Surgical treatment of CCFs is technically difficult and is associated with significant morbidity. Endovascular techniques from either an arterial or a venous approach have become the mainstay of treatment given the recent advances in endovascular technology. This article provides an overview of various endovascular approaches available for the treatment of CCFs.
This article provides an overview of direct and indirect carotid cavernous fistulas (CCFs) with emphasis on the recent advances in endovascular techniques available for treatment. It first briefly discusses the classification, etiology and pathology, clinical presentation, and diagnostic imaging of direct and indirect CCFs. Additionally, it provides a brief description of the medical management and surgical treatment of direct and indirect CCFs. The subsequent sections present a detailed discussion of the various endovascular techniques available for the treatment of direct and indirect CCFs with a brief discussion of the complications and results of treatment.
Classification
CCFs can be classified based on etiology (traumatic or spontaneous), rate of flow (high versus low flow), or the angiographic architecture (direct or indirect). The most commonly used classification scheme established by Barrow and colleagues divides the CCFs into four types, depending on the arterial supply. Direct (type A fistulas) are direct communications between the internal carotid artery (ICA) and the cavernous sinus, usually associated with high flow rates. Indirect fistulas (types B, C, and D) are dural arteriovenous fistulas (DAVFs) fed by the meningeal arteries of the ICA, the external carotid artery (ECA), or both. Type B fistulas are relatively uncommon and are supplied only by the dural branches of the ICA. Type C fistulas are supplied solely by the dural branches of the ECA. The most common indirect CCF is a type D fistula, which is supplied by the meningeal branches of both the ICA and ECA.
Etiology and pathology
Direct (type A) fistulas usually are traumatic, caused by motor vehicle accidents or penetrating injuries, and generally affect young males. Approximately 20% of type A CCFs may be spontaneous, resulting from the rupture of either a cavernous ICA aneurysm or a weakened ICA vessel wall coursing through the cavernous sinus.
Debrun described the site of CCF origin by dividing the cavernous ICA into five arbitrary segments. He found the most common site of occurrence is the proximal horizontal portion of the ICA near the artery of the inferior cavernous sinus (40%). CCFs occur with decreasing frequency at the junction of the horizontal and posterior vertical ascending cavernous segment (28%), at the origins from the posterior vertical ascending segment itself (20%), and distally near the anterior genu and the clinoidal segment (12%).
Van Dellen considers CCFs to be part of a continuum of injury to the arterial and venous vessel wall. An incomplete tear can result in a pseudoaneurysm of the cavernous carotid artery that can compress the venous sinusoids. A true CCF, however, occurs when the arterial tear is complete and there is associated breach of the walls of venous sinusoids.
Flow rates in type A fistulas are variable and depend on the size of the ostium and venous drainage. Complete steal of ICA flow to CCF occurs in approximately 5% of cases at diagnosis. Most fistula ostia measure 2.6 mm in diameter, typically small enough to be treated with detachable balloons with a mean volume of 0.28 cm 3 , equivalent to an inflated balloon dimension of 7 mm × 9 mm. Bilateral CCFs occur in approximately 1% to 2% of patients who have traumatic CCFs.
Spontaneous direct (type A) CCFs are more common in older women, although a few have been reported in children. They usually are caused by the rupture of a cavernous aneurysm or by the spontaneous rupture of a congenitally weakened, atherosclerotic, or diseased artery. Predisposition to spontaneous direct CCFs have been shown in fibromuscular dysplasia, Ehlers-Danlos syndrome, and pseudoxanthoma elasticum.
Indirect (types B, C, or D) CCFs have a predilection for spontaneous occurrence in postmenopausal women. Sinus thrombosis, hypertension, and diabetes have been suggested as predisposing factors. Trauma is less commonly associated with symptomatic indirect CCFs. Congenital indirect CCFs have been reported in children, including infants as young as 5 weeks of age.
The prevalence of types B, C, and D CCFs is uncertain: their frequency in a referral population is not representative of their occurrence in the general population.
Etiology and pathology
Direct (type A) fistulas usually are traumatic, caused by motor vehicle accidents or penetrating injuries, and generally affect young males. Approximately 20% of type A CCFs may be spontaneous, resulting from the rupture of either a cavernous ICA aneurysm or a weakened ICA vessel wall coursing through the cavernous sinus.
Debrun described the site of CCF origin by dividing the cavernous ICA into five arbitrary segments. He found the most common site of occurrence is the proximal horizontal portion of the ICA near the artery of the inferior cavernous sinus (40%). CCFs occur with decreasing frequency at the junction of the horizontal and posterior vertical ascending cavernous segment (28%), at the origins from the posterior vertical ascending segment itself (20%), and distally near the anterior genu and the clinoidal segment (12%).
Van Dellen considers CCFs to be part of a continuum of injury to the arterial and venous vessel wall. An incomplete tear can result in a pseudoaneurysm of the cavernous carotid artery that can compress the venous sinusoids. A true CCF, however, occurs when the arterial tear is complete and there is associated breach of the walls of venous sinusoids.
Flow rates in type A fistulas are variable and depend on the size of the ostium and venous drainage. Complete steal of ICA flow to CCF occurs in approximately 5% of cases at diagnosis. Most fistula ostia measure 2.6 mm in diameter, typically small enough to be treated with detachable balloons with a mean volume of 0.28 cm 3 , equivalent to an inflated balloon dimension of 7 mm × 9 mm. Bilateral CCFs occur in approximately 1% to 2% of patients who have traumatic CCFs.
Spontaneous direct (type A) CCFs are more common in older women, although a few have been reported in children. They usually are caused by the rupture of a cavernous aneurysm or by the spontaneous rupture of a congenitally weakened, atherosclerotic, or diseased artery. Predisposition to spontaneous direct CCFs have been shown in fibromuscular dysplasia, Ehlers-Danlos syndrome, and pseudoxanthoma elasticum.
Indirect (types B, C, or D) CCFs have a predilection for spontaneous occurrence in postmenopausal women. Sinus thrombosis, hypertension, and diabetes have been suggested as predisposing factors. Trauma is less commonly associated with symptomatic indirect CCFs. Congenital indirect CCFs have been reported in children, including infants as young as 5 weeks of age.
The prevalence of types B, C, and D CCFs is uncertain: their frequency in a referral population is not representative of their occurrence in the general population.
Clinical presentation
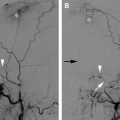
The classic presentation for a direct, high-flow CCF is the sudden development of a clinical triad: exophthalmos, bruit, and conjunctival chemosis. Direct CCFs can develop following a traumatic tear of the cavernous segment of the ICA and/or rupture of a cavernous ICA aneurysm. Complete disruption of the ICA wall allows highly pressurized arterial blood to be transmitted directly into the cavernous sinus and the ophthalmic veins, leading to venous hypertension. The manifestations of venous hypertension include ocular signs ( Fig. 1 ) and symptoms (proptosis, chemosis, conjunctival injection, cranial nerve pareses, and visual deficits), bleeding (from mouth, nose, or ears), and cerebral complications (intracranial hemorrhage, increased intracranial pressure, and steal phenomena). Five percent of patients develop intracranial hemorrhage, and 1% to 2% manifest life-threatening epistaxis. Epistaxis can be either acute or remote from the initial trauma caused by rupture of a pseudoaneurysmal cavernous sinus varix.
Compared with direct CCFs, indirect fistulas have a gradual onset, generally with a milder clinical presentation. Indirect fistulas usually are low-flow, acquired lesions that result from sinus thrombosis leading to venous congestion. Subsequently, abnormal arteriovenous shunting develops through the recanalized dural veins. DAVFs of the cavernous sinus often do not demonstrate the classic triad of symptoms. Patients who have these fistulas have chronically red eyes because of tortuous arterialization of the conjunctival veins. An ocular bruit may or may not be present with these lesions.
Unlike direct high-flow fistulas, most spontaneous indirect DAVFs improve, and many heal with medical management.
Diagnostic imaging
CT and MR imaging often are used in the initial work-up of a possible CCF. CT findings in CCFs include proptosis, enlargement of the extraocular muscles, enlargement and tortuosity of the superior ophthalmic vein, and enlargement of the ipsilateral cavernous sinus. MR imaging findings in CCFs are similar to those seen on CT with the addition of orbital edema and abnormal flow voids in the affected cavernous sinus. In the setting of a high-flow fistula and retrograde cortical venous reflux, MR or CT studies may reveal dilatation of leptomeningeal and cortical veins. In patients who have cerebral venous congestion and elevated intracranial pressures, cerebral edema and/or hemorrhage may be encountered.
Digital subtraction angiography is essential in confirming the diagnosis, classifying the fistula, and delineating the venous drainage pathways. Specifically, conventional angiography best characterizes the flow rate of the fistula and clearly distinguishes between direct and indirect fistulas (exact anatomic location of ICA tear versus dural ICA/ECA feeders). Moreover, it helps to assess the draining venous pathways (anterior versus posterior), cortical venous reflux, venous stenosis, or occlusions that could limit transvenous access to the cavernous sinus.
A complete and detailed diagnostic angiogram is recommended for planning either endovascular or surgical treatment. Selective ICA and ECA injections allow accurate classification of the fistula: direct fistula from the ICA or indirect dural fistula supplied by branches of the ICA/ECA. In addition, vertebral artery injections are helpful in fully appreciating the intracranial collateral circulation and circle of Willis in case ICA sacrifice must be considered as an option.
In evaluating direct CCFs, localizing the rent in the ICA can be challenging because of the high flow–related washout of intra-arterial contrast and instantaneous opacification of the cavernous sinus. Angiographic high-frame-rate imaging (> 5 frames/second) and rapid contrast injection rates (7 or 8 mL/second) may assist in evaluating the morphology of high-flow fistulas. If these techniques fail to identify the site of the fistulous communication accurately, specific maneuvers to decrease the flow rate across the fistula may be attempted. The Mehringer-Hieshima maneuver consists of injecting the ipsilateral ICA and manual compression of the ipsilateral common carotid artery while filming at a slower frame rate. Use of this maneuver slows the rate of opacification of the fistula and thereby allows better delineation of the fistula site. Another maneuver is the Huber maneuver, which involves injection of the ipsilateral vertebral artery with manual compression of the affected carotid artery. With this maneuver, the fistula is opacified through a posterior communicating artery if patent.
High-frame-rate imaging and magnified views of the head and neck allow detailed vascular mapping for anticipated transarterial and/or transvenous approaches. Delayed angiographic sequences incorporate the venous phase and help evaluate the patency of the major venous drainage pathways and the presence of cortical venous reflux and venous tortuosity and/or stenosis.
Medical management
Unlike high-flow direct CCFs, low-flow indirect or dural CCFs are not associated with increased mortality or significant risk for intracranial hemorrhage. Even in anterior draining fistulas that may lead to ocular manifestations, approximately 20% to 50% of dural CCFs heal spontaneously within days to months after symptomatic presentation. Therefore, an accepted practice is to treat the patient’s ocular symptoms medically with prism therapy or patching for diplopia, topical agents for elevated intraocular pressure, lubrication for proptosis-related keratopathy, and/or systemic corticosteroids if needed.
Furthermore, manual external carotid compression therapy may be initiated as a noninvasive treatment for indirect CCFs. This type of therapy is particularly effective in patients harboring fistulas in the anterior cavernous sinus and those who have relatively lower ocular pressures and a short interval between symptom onset and initiation of treatment. The patient is instructed to sit in a chair or lie in bed, compressing the carotid artery and jugular vein with the contralateral hand for a period of 10 seconds, four to six times each hour. Intermittent self-administered manual carotid-jugular compression alone can result in a cure in 30% of patients.
Contraindications to manual carotid compression include hypertensive carotid sinus syndrome, atherosclerotic stenosis, ulceration of the carotid artery, and a history of cerebral ischemia, because patients who have these anomalies cannot tolerate the transient occlusion of the ipsilateral ICA. The therapy must be discontinued if visual function shows progressive decline, the ocular pressure exceeds 25 mm Hg, or patients experience unbearable orbital pain.
Signs of ocular morbidity alter a conservative medical approach toward surgical or endovascular intervention. Patients who have progressive visual decline, diplopia, optic disc edema refractory to medication, proliferative retinopathy, increasing intraocular pressures, headaches, intraparenchymal hemorrhage, angiographic evidence of retrograde (cortical) venous drainage, or significant cosmetic deformity resulting from the fistula are offered definitive endovascular treatment.
Surgical treatment
Early treatment for CCF consisted of various surgical approaches. Trapping of the fistula by ligation of the cervical and intracranial ICA was described in the 1930s. Alternatively, carotid sacrifice was performed via embolization using different materials delivered by direct carotid exposure.
Although surgical trapping with ligation of the ICA is still considered an effective treatment for direct CCFs, sacrifice of the ICA is performed sparingly because of a significant risk of cerebral infarction even after successful balloon test occlusion studies. Furthermore, endovascular treatment now can offer similar results with a less invasive approach, avoiding the difficult surgical drilling needed for exposure of the clinoidal segment of the ICA.
In 1974, Parkison reported successful treatment of 9 of 11 patients by surgical exposure and packing of the cavernous sinus with preservation of the ICA. Although this surgical technique is useful for both direct and indirect CCFs, its role is limited if endovascular treatment can be performed because of its associated morbidity from cranial nerve deficits and residual fistulas.
Rarely, orbital surgery or decompression can be offered in cases that that do not respond to endovascular/surgical treatment or to those in which elevated intraocular pressures persist despite closure of the fistula.
Endovascular treatment
Recent advances in endovascular technology have made a number of different treatment options for CCFs currently available. The exact method chosen in each case depends on the anatomy of the fistula and operator/institutional preferences ( Fig. 2 ).
Direct fistulas occur from a tear in the cavernous segment of the ICA ( Fig. 2 A) or, less commonly, from the intracavernous rupture of an ICA aneurysm. The goal of treatment in direct CCFs is to occlude the site of communication between the ICA and the cavernous sinus while preserving the patency of the ICA. This goal can be accomplished with either transarterial obliteration of the fistula with a detachable balloon ( Figs. 2 B and 3 ), transarterial or transvenous obliteration of the ipsilateral cavernous sinus with coils or other embolic materials ( Fig. 2 C), or deployment of a covered stent across the fistula ( Fig. 2 D). Rarely, if the defect is large and cannot be repaired, the ICA may need to be sacrificed or trapped.
Indirect fistulas consist of small dural arteriovenous shunts between the meningeal branches of the ICA, the ECA, or both and the cavernous sinus. The goal of treatment in this condition is to interrupt the fistulous communications and decrease the pressure in the cavernous sinus. This goal can be accomplished by occluding the arterial branches supplying the fistula (transarterial embolization) or, more commonly, by occluding the cavernous sinus that harbors the fistulous communications (transvenous embolization).
The following sections provide a brief overview of the various endovascular options for the treatment of CCFs.
Transarterial Methods
In 1974, Serbinenko reported the first case of successful embolization of a CCF from an endovascular approach using a detachable balloon. Subsequently, Debrun and colleagues reported their successful treatment in 12 of 17 patients using detachable balloons. By the 1980s, detachable balloons were widely accepted as the treatment of choice for direct CCFs, although most balloons used in the United States were imported from abroad. The Food and Drug Administration (FDA) approved a detachable balloon system for peripheral vessel occlusion in 1981, but problems with the detachment system led to its withdrawal from the market in 1991. The first FDA-approved detachable balloon for intracranial use was not approved until 1998. Unfortunately, the balloon was removed from the United States market in 2003 because of problems with the valve mechanism, however, it continues to be available in many other parts of the world, however.
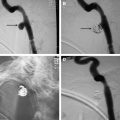
If available, the ideal treatment for a high-flow, direct CCF remains transarterial obliteration of the fistula with a detachable balloon. The balloon offers the advantage of being able to be flow-directed through the fistula and into the cavernous sinus. The balloon is inflated to a volume larger than the orifice of the fistula to prevent its retrograde prolapse into the ICA and then is detached. This approach constitutes an inexpensive, simple, but elegant endovascular treatment for direct CCFs (see Figs. 2 B and 3 ).
Occasionally, technical problems have been encountered with detachable balloon embolization, such as failure of flow-directed advancement from the ICA into the cavernous sinus or difficulty passing the balloon through the rent in the ICA. Additionally, early detachment/deflation of the balloon and occasional rupture of the balloon caused by contact with bone fragments have occurred.
Transarterial embolization with coils or other embolic material now is the mainstay of endovascular treatment for high-flow direct CCFs, given the unavailability of detachable balloons. Commonly used embolic agents include detachable platinum coils, n-butyl cyanoacrylate (n-BCA), and ethylene-vinyl alcohol copolymer (EVOH).
The standard transarterial approach consists of placing a guiding catheter in the cervical ICA. Next, a microcatheter is superselectively advanced into the cavernous segment of the ICA and across the tear into the cavernous sinus. Through this microcatheter, embolic material is placed into the cavernous sinus. The authors prefer to use detachable platinum coils because of their reliable and controlled deployment. The coils can be adjusted easily or even removed if the placement is not optimal. The use of transarterial liquid embolic agents such as n-BCA or EVOH to occlude direct CCFs also have been described. During transarterial embolization, a temporary balloon may be placed in the cavernous segment of the ICA (across the site of the tear) to protect the parent vessel and to prevent migration of the embolic material into the distal intracranial circulation.
CCFs caused by a small tear in the ICA can be treated with detachable balloons or coils as described. If there is a fairly large rent in the artery, however, the coils or balloon may herniate (or migrate) through the defect into the parent vessel. This retrograde herniation of embolic material can cause vessel occlusion and thromboembolic complications.
Recently, dedicated, intracranial, self-expanding stents have become available. These stents are approved by the FDA for coil embolization of wide-necked intracranial aneurysms. In the setting of direct CCFs, however, they can provide valuable scaffolding to reconstruct a severely injured intracranial ICA (see Fig. 2 C).
When deployed across a traumatic tear, stents create a barrier between the ICA and the cavernous sinus, preventing retrograde herniation of coils into the parent artery. These devices allow initial reconstruction of the damaged segment of the ICA and then controlled deposition of coils into the cavernous sinus through either a transarterial or transvenous approach. Using this technique, a direct CCF with severe injury to the ICA now can be occluded while preserving the ICA.
Direct CCFs caused by extensive injury to the ICA may not be amenable to endovascular occlusion with preservation of the parent artery. In these cases, occlusion of the arterial segment bearing the fistula may be the only viable option for treatment. If time permits, and the patient is cooperative, a temporary balloon test occlusion study of the involved ICA is recommended before permanent occlusion. Some risk of ischemic complications remain after ICA sacrifice despite a successful balloon test occlusion study.
To prevent retrograde flow from the supraclinoid ICA into the fistula, vessel occlusion is initiated cranial to the site of the suspected tear. Using multiple coils, the cavernous ICA then is occluded at the level of and caudal to the site of the fistula. This technique may be life saving in a patient who has extensive and unstable injuries. Recently, hydrogel-coated detachable coils have been introduced that expand on contact with blood, a favorable property especially in resilient high-flow fistulas that require high volumetric packing of the cavernous sinus. Furthermore, these coils may facilitate vessel sacrifice, decreasing the procedure and fluoroscopy times. Use of a vascular plug for arterial sacrifice also has been reported. Although the plug is easy to deploy and is effective, the navigation of the vascular plug into the distal ICA is currently difficult.
Placement of a stent or graft covered with polyfluorotetraethylene is an alternative treatment option if ICA sacrifice is not desired or is unacceptable because of an unsuccessful balloon test occlusion study ( Figs. 2 D and 4 ). Covered stent grafts can be extremely useful for the immediate obliteration of a direct CCF and other fistulas. Additionally, they may decrease the risk of ischemic stroke by preserving the involved artery while simultaneously sealing the site of the fistula. A few reports have described the successful use of a covered stent graft for the treatment of CCFs. Currently, the FDA has not approved any covered stent for intracranial use in the United States. The disadvantages of the stents include stiff and high-profile construction that make it difficult to navigate them into the distal ICA, risk of endoleaks, possibility of coverage of vital perforators, and lack of long-term safety data.