An epidural steroid injection (ESI) is a minimally invasive, image guided procedure for the treatment of back pain. Pain originating in the lumbar spine is the most common referral for ESI but the entire spine may be targeted. ESI can provide temporary but meaningful relief for patients who may have failed conservative management with oral analgesics and physical therapy. ESI may provide analgesia and anti-inflammatory effects that allow more conservative measures like physical therapy to become more effective. ESI also serves as a bridge between conservative and surgical management, intervention for postsurgical pain, or an alternative for nonsurgical candidates. This article reviews the technique for performing ESI in the cervical, thoracic, and lumbosacral spine.
Clinical evaluation of the patient
Epidural steroid injection (ESI) can provide temporary pain relief in patients experiencing significant and often debilitating back pain. The causes are predominately degenerative in nature from intervertebral disc herniation, osteophyte formation, facet joint arthropathy, or ligamentum flavum hypertrophy. Despite multiple anatomic causes the outcome is the same, a nerve is compressed by a fixed structure resulting in intraneural edema and possible nerve demyelination. Referral to radiology for ESI often comes from patients who have already been seen by another subspecialist and previously been deemed a candidate for the procedure. However, it is important for the radiology interventionalist to evaluate the patient independently as well as in conjunction with any prior workup.
Patient history is key in selecting both a treatment site and needle approach in the procedural suite. A basic series of questions will lead you to their problematic spinal level. Localization of pain to a specific area versus a generalized region may alter your approach. Transforaminal access is spinal level specific while interlaminar access allows for injection agent diffusion over multiple spinal levels. Laterality is an obvious but important aspect as some patients may not localize to just 1 side. Radiation of the pain down an arm or leg will typically follow a dermatomal fashion that can be used for judging both spinal level and severity of nerve involvement. A bulging disc, hypertrophied facet, or enlarged ligamentum flavum abutting the nerve is enough to cause pain. More severe compression of the nerve may result in radiculopathy or neural degeneration manifesting as numbness or weakness within the dermatome. The characteristics of the pain can help you predict severity of nerve involvement for preprocedure counseling of ESI efficacy and duration of expected relief.
Finally, a key final step in the patient history is management of expectations. While most patients do find significant relief following ESI, it is important to discuss with the patient that ESI will provide relief if intraneural inflammation and edema is the major exacerbating factor of their pain. If inflammation is not a significant pain contributor, the patient may experience transient or nonexistant analgesic effects. The radiologist may be the first person to explain that the goal of ESI is noncurative but 3-4 months of pain reduction with an expected return of symptoms. Setting expectations gives the patient a timeline to monitor for symptom recurrence and prevents unintentional, unrealistic goals for the procedure.
Physical examination has limited utility in the evaluation of radiculopathy and nerve related back pain. Some maneuvers from the patient’s history such as relief with leaning forward may elicit findings suggestive of spinal canal stenosis. Overall, history and imaging are better suited for identification and characterization of the patient’s pain.
Imaging is the cornerstone for both localization of symptoms and preprocedural planning. Computed tomography (CT) or magnetic resonance imaging (MRI) of the spine should ideally be performed before the patient is referred to radiology for ESI. While ESI can be performed without preprocedural imaging, this is not preferred due to lessened efficacy if the appropriate level is not targeted and variables that may make for a more challenging procedure such as osteophytic projections or complete neuroforaminal narrowing are not identified. There is a dichotomy in imaging inspection and the patient interview: reviewing the images before obtaining a patient history or after. Each method does hold merit and may work better for differing clinical workflows, but our recommendation is to review the CT or MRI after interviewing the patient. Just as a detailed imaging indication provided by a clinician can assist in image interpretation, so too can your history assist in image interpretation for procedural consideration. Reviewing of imaging following a patient interview can retrospectively elicit findings not evident prospectively or deemed insignificant on initial interpretation.
Preprocedural laboratory values are an important consideration for the proceduralist. According to the Society of Interventional Radiology (SIR), ESI is considered a high bleeding risk procedure given the proximity of epidural fat and nerve roots to spinal venous plexuses and arterial structures. We recommend routinely obtaining an international normalized ratio (INR) and platelets with goal INR ≤1.8 and platelets ≥50,000 according to the SIR procedural guidelines.
Indications and contraindications
ESI is indicated for the relief of back pain with or without radicular features. Injections can be done throughout the spine but are predominantly done in the cervical and lumbar segments. Spinal canal stenosis is also a common indication for ESI but delineation from cauda equina syndrome is warranted as ESI will not benefit patients in the latter group. Axial back pain or pain relieved by spine decompression is also a candidate for ESI. Patients who have recurrent back pain in the postsurgical or postvertebral augmentation setting can also undergo ESI.
Absolute contraindications to ESI include active systemic infection, spondylodiscitis, irreversible coagulopathy, and anaphylactic contrast allergy. The procedure can be performed without contrast injection for needle placement confirmation but this is not preferred. Relative contraindications may include technical feasibility of the procedure, patient inability to lay prone, pregnancy, or malignancy at the injection site. Another frequently encountered relative contraindication is diabetes mellitus in the setting of peri-procedural steroid injection. Although, diabetes is typically a nonissue if it is well-controlled. As an elective procedure, delay the injection as needed to prevent unwanted complications.
Another consideration for deferring the ESI procedure to a later date is failure to discontinue anticoagulation. We recommend following the SIR procedural guidelines for holding anticoagulation, although institutional and interventionalist criteria may vary. Frequently, we use the SIR Guidelines mobile application to assist in procedural planning. The following recommendations are taken directly from the SIR mobile app and guidelines: antiplatelet therapy (e.g., aspirin, clopidogrel) and warfarin should be held for 5 days preprocedurally. Therapeutic dose enoxaparin should be held for 1 day preprocedurally. Direct acting oral anticoagulation should be held for 2-3 days preprocedurally depending on the patient’s renal function.
From a workflow standpoint, these contraindications should be identified well before the patient is on the imaging table. This further underscores the importance of a careful patient interview before undergoing an ESI.
Equipment and materials
A standard spinal needle tray for lumbar puncture can be utilized for ESI. Fluoroscopic guidance is the most popular option given its availability and real time image acquisition. A multiplanar fluoroscope is not technically required but highly recommended for the ability to obtain orthogonal views. Many institutions are also able to offer a procedural CT machine for ESI. The benefits of a stepwise CT guided ESI can be especially useful in patients with difficult anatomy or pathology adjacent to the injection pathway. However, CT lacks real-time imaging for needle adjustments. Additionally, if an intravascular injection is made, the contrast may wash away before the operator obtains the next image sequence. Ultrasound guidance is also an option but highly operator and patient body habitus dependent. In practice, ultrasound is typically utilized for special cases such as pregnancy or significant postsurgical metal artifact.
For contrast medium selection, consider the intrathecal implications of injection. As such, myelogram safe contrast material is required for ESI. Myelogram contrast is nonionic and only certain concentrations are approved for intrathecal use such as iohexol 180, 240, or 300.
Injection agent medications and dosing is widely variable depending on the interventionalist, institution, and formulary. The mainstays of injected material are a steroid agent mixed with a local anesthetic. Steroid selection can be subdivided into particulate (e.g., triamcinolone, methylprednisolone, prednisolone) and nonparticulate (e.g., dexamethasone) agents. Particulate agents are not taken up by cells and have a theoretical longer lasting anti-inflammatory and analgesic effect. However, there are rare complications where particulate steroids aggregate when injected intra-arterially resulting in embolization of neurovasculature and catastrophic neurologic sequelae. Nonparticulate agents do not aggregate and offer a virtually nonexistent embolic potential if intravascular injection occurs. Additionally, nonparticulate agents have few systemic implications. Betamethasone is a commonly used ESI agent which has both particulate and nonparticulate steroid properties. Local anesthetics include lidocaine, bupivacaine, and ropivacaine. Bupivacaine is the preferred agent due to its higher potency and longer duration of action compared to lidocaine. Ropivacaine has been shown to have similar potency to bupivacaine. Of note, when injections are performed at and above the level of C6, steroid injection alone is recommended. Anesthetic injection at these levels poses risk of diaphragmatic paralysis.
There is no gold standard for dosing volumes of anesthetic and steroid mixtures. The dosing reflected below is based on author preferences and institutional norms. In the cervical spine, 1 cc of betamethasone 8 mg. In the thoracic spine, 2 cc of betamethasone 8 mg mixed with 3-5 cc of bupivacaine. In the lumbar spine, 2 cc of betamethasone 8 mg mixed with 5-7 cc of bupivacaine. In the sacral hiatus approach, 2 cc of betamethasone 8 mg mixed with 3-5 cc of bupivacaine.
Technique
General
Once the patient has undergone preprocedural determination of the target spinal level, the operator should identify vascular and neural structures at the level of concern as well as 1 level above and below the target level. In the event of dural penetration, the needle can be repositioned to a different level for analgesia, although, in a less-specific fashion. Steroid should not be injected at a level where the thecal sac was accessed. Ensure the patient has discontinued appropriate anticoagulants and is not actively taking antibiotics for an infection.
Place the patient on the procedural table. Identify the access site radiographically with the tip of a towel clamp or Kelly forceps and mark the skin. Sterilize, prepare, drape, and anesthetize the access site according to institutional protocol.
Lumbar
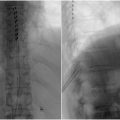
For the lumbar interlaminar approach ( Fig. 1 ), prone the patient and position the image intensifier in the anterior-posterior (AP) orientation. To increase the size of the interlaminar space, angle the image intensifier caudally towards the patients feet by approximately 15 degrees. Of note, the needle will also need to be angled accordingly to maintain the “bulls eye” needle appearance. Advance the needle into the interlaminar space directed at the bases of the spinous processes where the dorsal fat pad is typically the most thick. Once through the ligamentum flavum there will be the characteristic decrease in force required to advance the needle similar to a lumbar puncture. Confirm needle placement using lateral views of the spine which should show the tip at the base of the spinous process.