(1)
Princess Elizabeth Hospital Le Vauquiedor St. Martin’s Guernsey, Channel Islands, UK
(2)
Brighton and Sussex Medical School, Brighton, England UK
Abstract
The normal breast tissue consists of branching ducts, which terminate into the secretory lobular units. The ducts and lobules are lined by two layers of epithelium consisting of the inner secretory epithelial layer and the outer myoepithelial layer. An increase in the number of epithelial cells, three to four cells above the basement membrane, without bridging or distension of the lumina, indicates mild epithelial hyperplasia (Fitzgibbons et al. 1998). Mild epithelial hyperplasia is not associated with increased risk of malignancy (Rogers 1987). This pattern of mild epithelial proliferation is present in most specimens excised for benign and malignant breast tissue.
Learning Points
UDH is a common finding in specimens excised for benign disease or breast cancer.
ADH is difficult to differentiate from low-grade DCIS resulting in high intra-and interobserver variation on reporting.
Diagnosis of ADH in the needle core should lead to excision biopsy to exclude high-grade lesions.
There is no consensus on how to manage ADH on the margin in a lumpectomy specimen; therefore, MDT discussion is important.
Lobular neoplasia (LN) should be excised if present on needle core to exclude high-grade lesions.
There is no consensus regarding risk of bilateral cancer in patients with LN.
LN should be managed conservatively if it is an incidental finding in a specimen excised for another lesion.
Molecular studies indicate that LN is both a risk marker and non-obligate precursor of invasive carcinoma.
Intraductal papillomas can arise centrally or at the periphery of the breast.
Diagnosis of a papillary lesion on biopsy should prompt an excisional biopsy as a high-grade papillary neoplasm cannot be excluded.
Intraductal papillomas behave as both a marker and precursor of malignancy.
The diagnosis of pregnancy-like changes should not be made in a lactating breast.
10.1 Usual Ductal Hyperplasia (UDH)
The normal breast tissue consists of branching ducts, which terminate into the secretory lobular units. The ducts and lobules are lined by two layers of epithelium consisting of the inner secretory epithelial layer and the outer myoepithelial layer. An increase in the number of epithelial cells, three to four cells above the basement membrane, without bridging or distension of the lumina, indicates mild epithelial hyperplasia (Fitzgibbons et al. 1998). Mild epithelial hyperplasia is not associated with increased risk of malignancy (Rogers 1987). This pattern of mild epithelial proliferation is present in most specimens excised for benign and malignant breast tissue.
Usual ductal hyperplasia (UDH) denotes a step further in the proliferation of breast epithelium and is diagnosed by the presence of four or more cell layers above the basement membrane. This proliferation is also termed hyperplasia of usual type (HUT) moderate epithelial hyperplasia without atypia, proliferative disease without atypia (PDWA), regular epithelial hyperplasia or ordinary epithelial hyperplasia. The term ‘usual’ is intended to emphasise the fact that the epithelial proliferation is the type commonly present when the number of cells is increased within a basement membrane-bound space (Page 1992). Use of terms such as epitheliosis and papillomatosis should be avoided when describing epithelial hyperplasia, as they are confusing and non-specific.
UDH is usually present in specimens excised for malignant or benign breast lesions. The latter include fibrocystic change, radial scars, atypical hyperplasia, fibroadenomas or intraductal papillomas. Any of these lesions can be symptomatic or screen detected. It is unusual for mild or moderate epithelial hyperplasia without atypia to be the main mammographic abnormality to prompt an excisional biopsy, but occasionally calcification can lead to mammographic detection. UDH is architecturally heterogeneous, which could make histological classification difficult.
10.1.1 Pathological Features of UDH
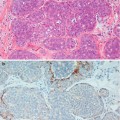
The intraductal proliferation can exhibit interconnecting arcades with false glandular lumina, solid proliferation or micropapillary structures (Fig. 10.1). Apocrine cells may also be present. Despite the architectural heterogeneity, the hallmark of UDH is the lack of cytological atypia. The proliferation invariably shows a mixture of epithelial and myoepithelial cells. Frequently, immunocytochemistry markers are applied as an adjunct to conventional haematoxylin and eosin staining in the differential diagnosis of hyperplastic or neoplastic proliferation. Cytokeratins 5 and 6 (CK5/6) have been found to be useful in differentiating hyperplastic from neoplastic proliferations of the breast by highlighting myoepithelial (basal) cells which are present in benign but not neoplastic proliferations (Otterbach et al. 2000; Boecker et al. 2001).
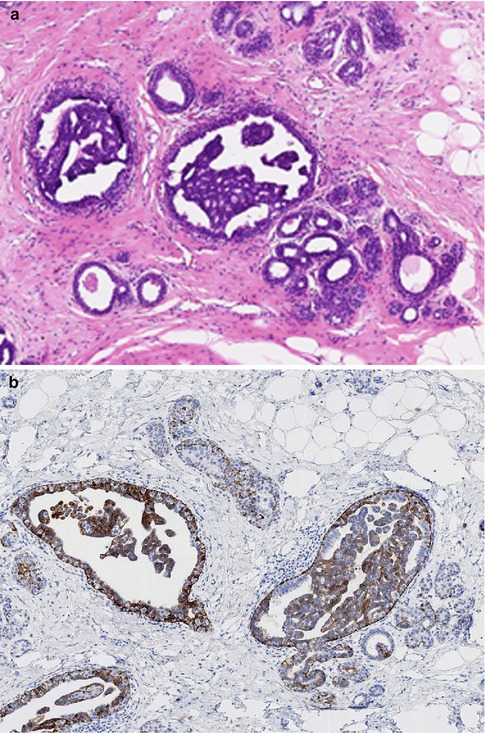
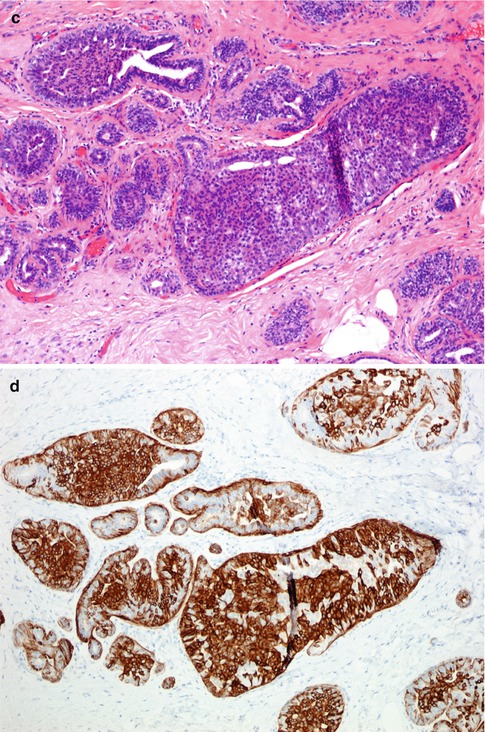


Fig. 10.1
(a) Usual ductal hyperplasia (UDH) consisting of epithelial proliferation with false lumina. (b) Staining with CK5/6 confirms a benign proliferation. (c) UDH with solid proliferation. (d) Staining with CK5/6 confirms a benign proliferation
It has been generally assumed that in the pathogenesis of breast cancer, there is a progressive intraductal epithelial proliferation from UDH through ADH to DCIS and subsequent invasive cancer. This concept has been challenged. Using double immunofluorescence analysis and Western blotting, Boecker et al. (2002) identified a third cell type within the normal breast tissue, which expresses cytokeratin 5 (CK5) only. The authors claim that this CK5-positive cell represents the adult stem cell, which gives rise to glandular and myoepithelial cell lineage. Double staining revealed UDH and ADH and DCIS were phenotypically different. UDH expressed CK5, whereas ADH and DCIS were positive for CK8/18/19 but negative for CK5. This study considered the CK5-positive cell in epithelial hyperplasia of usual type as the progenitor or committed stem cell, which proliferates into glandular cells via intermediate stages. The results of this double-labelling technique suggest that UDH is unlikely to be an ‘obligate’ precursor of DCIS. Shaaban et al. (2003) reported progressive decline in the expression of oestrogen receptor-β (ER-β) from normal epithelium, UDH, DCIS and invasive carcinoma. ER-β expression was significantly lower in UDH than normal lobules. The authors postulated that ER-β protein downregulates promotion of neoplastic progression in benign lesions of the breast. In a separate study, Mao et al. (2010) reported 100 % expression of ER-α in 77 cases of UDH and none of the cases expressed p53 protein.
10.1.2 Genetic Alterations in UDH
O’Connell and colleagues (1998) investigated loss of heterozygosity (LOH) at 15 genetic loci including 16p, 17p and 17q in 399 putative precursors of invasive breast cancer, which consisted of 211 UDH, 51 ADH, 81 non-comedo DCIS and 56 comedo DCIS. Although the prevalence of LOH was low in epithelial hyperplasias from non-cancerous breasts, they reported high LOH in tissue harvested from cancerous breasts in 37 % of UDH, 45 % of ADH, 77 % of non-comedo DCIS and 80 % of comedo DCIS. These lesions shared LOH with synchronous cancers at one or more loci, supporting the theory that the putative precursors and the cancers were genetically related. Lakhani et al. (1996) also detected allelic imbalance in UDH, suggesting that the proliferations were clonal and possibly neoplastic. Clonality in some cases of UDH was also reported using comparative genomic hybridisation (CGH) (Jones et al. 2003). This study reported, among others, loss of material on chromosomes 1p, 16p, 17q and 22p, which are the common loci for genetic alteration in invasive breast cancer. In a previous study also applying CGH, Gong et al. (2001) reported chromosome copy number alterations on 16q (five cases) and 17p (two cases) in UDH and ADH in cases where these lesions occurred concurrently. However, only one out of nine cases of pure UDH showed CGH abnormalities.
Further molecular studies to clarify the role of UDH in the pathogenesis of breast cancer were carried out by Xu and colleagues (2008) when they investigated chromosomal imbalances and clonality by using CGH. The mean value of chromosomal alteration was 1.95 (39/20) in UDH, 9.5 (19/2) in ADH, 11.0 (33/3) in DCIS and 18.2 (89/5) in invasive ductal carcinoma. Some common deletions were in chromosomes 1p, 13q and 16q with high frequency of amplification in 1q, 3p, 6p, 11q, 12q, 13q, 16p, 17q and 20q. The results revealed that the deletion of DNA copy was lowest in UDH with a linear increase in ADH, DCIS and IDC (invasive ductal carcinoma). In this study, a significant number of UDH shared common genetic alterations with ADH, DCIS and IDC suggesting that UDH was a putative precursor of ductal carcinoma. These results are in conflict with Boecker and colleagues’ (2001) findings using immunocytochemistry staining.
10.1.3 UDH as Risk Factor of Subsequent Malignancy
When Dupont and Page (1985) followed up women aged between 20 and 55 years with PDWA, they reported a RR of subsequent cancer of 1.9 times that of the general population. The presence of family history of breast cancer elevated the risk to 3.2. Women over 55 years of age with PDWA had a RR of 2.2 times that of women without proliferative disease. The presence of calcification plus PDWA in women over 55 years of age was associated with an RR of 5.6 compared to women of the same age group without proliferative disease. In an autopsy study, Kramer and Rush (1973) examined breast tissue from 70 women who were over the age of 70 and found intraductal epithelial hyperplasia in 69 % of cases and 43 % of the lesions were considered severe. These two studies suggest that significant epithelial hyperplasia is more prevalent in older women than younger women.
Tavassoli and Norris (1990) followed up 117 women (age range 15–75) with ordinary or regular intraductal epithelial hyperplasia for 4.5–25.7 years (median, 14 years). They recorded regular or ordinary epithelial hyperplasia if two ducts, ductules or transformed lobules were involved. The proliferation exhibited tufts, bridges, arcades, layers and irregular and peripheral fenestrations with or without streaming of cells. The cell population consisted of myoepithelial, epithelial and apocrine metaplastic cells. Apocrine cells were present in 102 of the 117 biopsies and 83 biopsies had apocrine hyperplasia. Sclerosing adenosis was present in 80 biopsies and calcification in 20 biopsies. During follow-up, invasive carcinoma developed in three women in the ipsilateral breast, ductal carcinoma in situ (DCIS) developed in the ipsilateral breast in one woman and in the contralateral breasts in two women. One of the women with combined regular intraductal hyperplasia, sclerosing adenosis and calcification also developed carcinoma. Atypical intraductal hyperplasia developed in the ipsilateral breasts in five patients and in the contralateral breast in one patient. The prevalence of cancer associated with regular intraductal epithelial hyperplasia was only 2.6 % at 14 years compared to 9.8 % at 8 years in women with atypical ductal hyperplasia (ADH) in the same study. This study also highlighted the importance of age when considering the risk of subsequent malignancy. Carcinoma did not develop in women below the age of 30 who had regular epithelial hyperplasia in their biopsies, whereas carcinoma subsequently developed in 1.9 % of the women between 31and 45 years of age and in 3.8 % of the women over 45 years of age. In contrast, an average of 10 % of women with ADH in the same study developed carcinoma in the three age groups (< 30, 31–45, > 45). The average interval for developing subsequent carcinoma was similar, 8.3 years in women with regular intraductal hyperplasia and 8.8 years in women with ADH.
The report by Tavassoli and Norris (1990) highlights the difficulties in assessing mixed epithelial proliferations and in determining which of these is clinically significant. However, assessing UDH is less controversial than assessing ADH. In several studies that assessed the risk of UDH with regard to progression to invasive malignancy, there was overall concordance in the results obtained (Table 10.1). Unlike ADH, a diagnosis of UDH does not warrant intensive follow-up. Based on these reports, if UDH is the only abnormality in a needle core biopsy, this does not require surgical excision, unless there is clinical, radiological discordance. These patients do not require chemoprevention or regular screening (Kiluk et al. 2007). However, as the above studies have demonstrated, epithelial hyperplasia is more prevalent in older women with a higher risk of cancer when compared with younger women.
10.2 Atypical Ductal Hyperplasia (ADH)
10.2.1 The Concept of Atypical Ductal Hyperplasia
Although pathologists recognised a borderline lesion for a long time, the concept of atypical hyperplasia (ADH) implying a possible risk of subsequent malignancy or a premalignant lesion was not universally acceptable (Gallagher and Martin 1969; Black et al. 1972; Ashikari et al. 1974). Azzopardi (1979) felt strongly that the term atypical hyperplasia was inappropriate and believed this would ‘frighten surgeons into performing unnecessary mastectomies’. With the current multidisciplinary team approach to patient care, the risk of the surgeon making single-handled decisions should be rare.
The histological diagnosis of ADH is fraught with inconsistencies because of marked intraobserver and interobserver variation among pathologists when classifying this lesion (Rosai 1991; Sloane et al. 1994; Jain et al. 2011). Black and Chabon (1969) classified intraductal epithelial proliferations into five grades as follows: 1, normal; 2, hyperplasia; 3, distinct but minimal atypia; 4, atypia suggestive of carcinoma in situ; and 5, atypia consistent with carcinoma in situ. Although this five-tier grading was replaced by the three grades of Page and Rogers (1992), it would have been useful to maintain the minimal atypia grade of Black and Chabon, as most pathologists have discovered that there are epithelial proliferations that show cytological atypia without sufficient architectural changes for the lesion to be accommodated into the ADH or DCIS categories.
Tavassoli and Norris (1990) defined atypical hyperplasia as a lesion having ‘cytological and architectural features of non-necrotic intraductal carcinoma and the changes may involve two or more ductules, but the involved ducts or ductules should measure less than 2 mm in diameter’. The criterion of ADH proposed by Fechner and Mills (1990) was ‘when there is partial involvement of a duct by alterations architecturally and cytologically indistinguishable from cribriform carcinoma’. Rogers (1987) deemed a lesion to exhibit features of ADH ‘when either cytological or pattern criteria of DCIS are met, but both are not present in full flower’. It is this lack of a concrete definition that made and still makes the accurate diagnosis of ADH difficult and results in the variation of the level of risk of subsequent breast cancer attributed to ADH. One typical example is the so-called clinging carcinoma of Azzopardi (1979), which was classified as a variant of DCIS, whereas the UK National Health Service Breast Screening Programme classified this lesion as ADH (NHSBSP 1997). This lesion is now classified as flat epithelial atypia (FEA); see Chap. 11.
10.2.2 Radiological Features of ADH
ADH is rare as a sole symptomatic breast lesion but is reported frequently in mammographically detected lesions with variable prevalence of between 7 % and 10 % (Sneige et al. 2003; Rubin et al. 1988). In one of the largest series to be reported, ADH was identified in only 3.6 % of more than 10,000 biopsies excised for symptomatic breast disease (Dupont and Page 1985). Although ADH can be detected mammographically as the dominant lesion due to the presence of microcalcification (Sneige et al. 2003), in the majority of cases, there are other associated lesions such as DCIS, fibrocystic change, radial scars or intraductal papillomas.
Rubin et al. (1993) reviewed the mammographic and histological features of 21 patients with atypical hyperplasia (ductal, lobular or mixed), 40 patients with DCIS and 14 patients with DCIS plus micro-invasion. The mean age of patients with atypical hyperplasia was 54.7 years (range 41–79 years), compared with 57.4 years (range 29–85 years) for patients with DCIS and 60.6 years (range 37–88 years) for patients with DCIS plus micro-invasion. These findings suggest that atypical hyperplasia was more frequent in younger women than older women, implying an age-related progression of epithelial proliferation to overt malignancy. Calcification was detected mammographically in 59 % of cases of atypical hyperplasia, 68 % DCIS and 79 % DCIS and micro-invasion. Other mammographic abnormalities associated with atypical hyperplasia included: parenchymal distortion (23 %), mass lesion (9 %) and no abnormality (9 %). On histological examination, calcification was present in 95 % of cases of atypical hyperplasia. The calcification was present in atypical hyperplasia or DCIS and the surrounding benign breast tissue in 20 %, benign tissue only in 75 % and other calcification in 5 % of the cases. This highlights the fact that calcification noted at histological examination does not necessarily represent the mammographic abnormality and radio-pathological correlation is essential to achieve appropriate patient management. Rubin and colleagues (1993) confirmed previous reports that there are no radiological pathognomonic features of atypical hyperplasia. The authors reported parenchymal distortion and calcification noted mammographically, which led to the diagnosis of atypical hyperplasia, was associated with other benign proliferative diseases rather than specific to atypical hyperplasia. Furthermore in 82 % of cases of atypical hyperplasia, the foci were less than 5 mm in size and this was unlikely to produce a mammographic abnormality. However, 71 % of the women with atypical hyperplasia had dense breasts (Wolfe Category P2 or DY, see Chap. 16; Wolfe 1976), compared with 60 % with DCIS and 50 % with DCIS plus micro-invasion. The mammographically dense breasts may reflect the young age of the women.
In a separate study, Helvie and colleagues (1991) reviewed mammograms and histological slides of 58 patients with a diagnosis of atypical hyperplasia. The authors reported 41 % (24/58) concordance between mammographic and histological features of ADH, mostly due to the presence of clustered microcalcification. However, the features were not pathognomonic.
10.2.3 Pathological Features of ADH
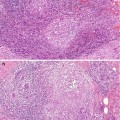
ADH is a borderline intraductal proliferation, with a potential to progress to DCIS. ADH is particularly difficult to distinguish from low-grade DCIS with a cribriform or micropapillary pattern. Page and Rogers (1992) set out criteria to assist in the diagnosis of ADH, which are based on the architectural and cytological features and the size of the lesion. By applying these criteria, a diagnosis of ADH should be made when there is partial involvement of two-membrane-bound spaces by a population of atypical cells similar to those seen in non-comedo DCIS. Although ADH can be detected as the dominant lesion mammographically, usually this is associated with DCIS or another high-grade lesion (Fig. 10.2). The atypical cells are usually polarised and are present above the basement membrane. Previously, Tavassoli and Norris (1990) assessed the anatomic extent of atypical hyperplasia and applied a size limit of less than 3 mm for an intraductal proliferation to qualify for the diagnosis of ADH. The rationale for this was that there are some ducts larger than 10 mm, which may contain highly atypical neoplastic cells, and one should not hesitate to render a diagnosis of intraductal carcinoma in these circumstances. The UK National Health Service Breast Screening Programme (NHSBSP 1997) advocates that the diagnosis of ADH should be made if the diagnosis of DCIS is seriously being considered and the lesion measures between 2 and 3 mm in diameter. The criteria that differentiate UDH, ADH and low-grade DCIS are set out in Table 10.2.
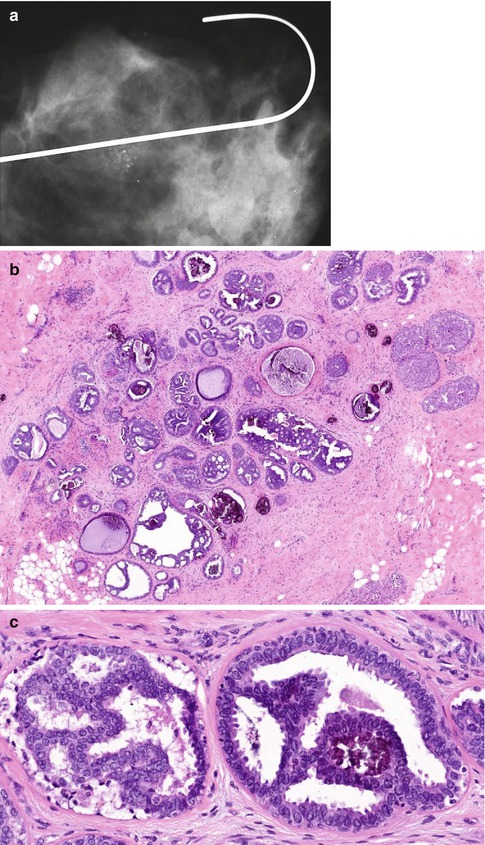
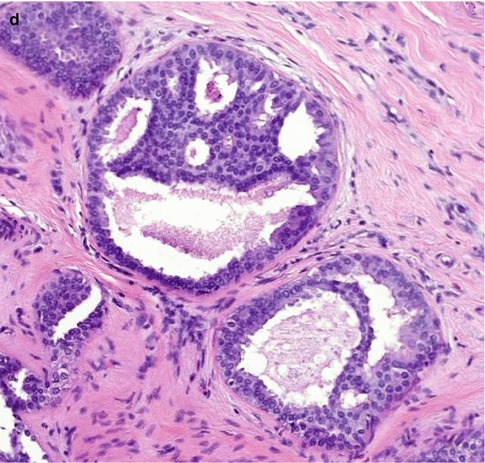


Fig. 10.2
(a) This specimen X-ray shows a focus of mammographically detected indeterminate microcalcification (R3). The needle core biopsy was not diagnostic. (b) The main lesion in the excision biopsy was low-grade DCIS with a predominantly cribriform pattern, which measured 5 mm in diameter. (c) There are also ducts showing features of ADH and this demonstrates how difficult it is to differentiate ADH from low-grade DCIS. In this field, the calcification is present ADH. (d) This field shows non-calcifying ADH with central monomorphic cells creating cribriform and bridging patterns
Table 10.2
A comparison of histological features of ductal hyperplasia and DCIS
Histological features | Usual type ductal hyperplasia | Atypical ductal hyperplasia | Low nuclear grade DCIS |
|---|---|---|---|
Size | Variable size but rarely extensive unless associated with other benign processes such as papilloma or radial scar | Usually small (less than 2–3 mm) unless associated with other benign processes such as papilloma or radial scar | Rarely less than 2–3 mm and may be very extensive |
Cellular composition | Mixed. Epithelial cell and spindle-shaped cells a present. Lymphocytes and macrophages may also be present. Myoepithelial hyperplasia may occur around the periphery | May be uniform cell population but merges with areas of usual type hyperplasia within the same duct space. Spindle-shaped cells may be intermingled with the proliferating cells | Single-cell population. Spindle-shaped cells not seen. Myoepithelial cells usually in normal location around duct periphery but may be attenuated |
Architecture | Variable | Micropapillary, cribriform or solid patterns but may be rudimentary | Well-developed micropapillary, cribriform or solid patterns |
Lumina | Irregular, often ill-defined peripheral slit-like spaces are common and a useful distinguishing feature | May be distinct, well-formed rounded spaces in cribriform type. Irregular, ill-defined lumina may also be present | Well-delineated, regular punched-out lumina in cribriform type |
Cell orientation | Often streaming pattern with long axes of nuclei arranged parallel to direction of cellular bridges, which often have a ‘tapering’ appearance | Cell nuclei may be at right angles to bridges in cribriform types, forming ‘rigid’ structures | Micropapillary structures with indiscernible fibrovascular cores or smooth, well-delineated geometric spaces. Cell bridges ‘rigid’ in cribriform type with nuclei orientated towards the luminal space |
Nuclear spacing | Uneven | May be even or uneven | Even |
Epithelial/tumour cell character | Small ovoid but showing variation in shape | Small uniform or medium-sized monotonous cell population present at least focally | Small uniform monotonous cell population |
Nucleoli | Indistinct | Single small | Single small |
Mitoses | Infrequent with no abnormal forms | Infrequent, abnormal forms rare | Infrequent, abnormal forms rare |
Necrosis | Rare | Rare | If present, confined to small particulate debris in cribriform and/or luminal spaces |
10.2.4 Genetic Alterations in ADH
Breast cancer is thought to develop from normal epithelium through the following sequence: UDH → ADH → DCIS → IDC. Unlike in Vogelstein’s model of colorectal cancer (1988), genetic abnormalities are not mirrored by corresponding phenotypic changes. Aubele et al. (2000) carried out a study of lumpectomy specimens from five patients using laser microdissection of areas of ductal hyperplasia without atypia, ADH, DCIS and IDC for correlation of genotypic and phenotypic changes. The results revealed an increasing mean number of chromosomal abnormalities (gains and losses), with an increase in the histological severity of the disease process. Chromosomal changes found in each of the four histological proliferation included gains on 10q, 12q, 16p and 20q and loss on 13q. In ductal hyperplasia without atypia, gain on 20q as well as loss on 13q was detected in high frequency (four out of five samples). Alteration identified in more than 50 % of ADH samples included gains on 3p, 8q, 15q and 22q and loss on 16q. In DCIS, gains of DNA on 1q and 17q and loss on 4q were additionally found, and in IDC, further gains on 6p, 10q, 11q and 17p were identified. These chromosomal alterations suggest that the regions harbour tumour suppressor genes or oncogenes significant for the development of ductal carcinoma of the breast. In a separate study using tissue from nine patients with ADH and adjacent UDH and nine patients with pure UDH, Gong et al. (2001) reported loss of 16q in five cases of ADH and loss of 17p in two cases of ADH. These chromosomal abnormalities were also demonstrated in the adjacent UDH. Only one of the cases of pure UDH showed chromosomal abnormalities. This data suggest that UDH may represent a precursor lesion of ADH. In a different study, loss of heterozygosity (LOH) in invasive cancer on chromosome 2p, 11p and 17q was shown in 37 % cases of UDH, 45 % of ADH, 77 % non-comedo DCIS and 80 % of comedo DCIS supporting the notion that the putative precursors associated with the invasive cancer were genetically related (O’Connell et al. 1998) (Table 10.3).
Table 10.3
A comparison of ALH and ADH with emphasis on RR value
Criterion | ALH | ADH |
|---|---|---|
Radiological features | None | +/− calcification |
Breast at risk of cancer | Both, but mostly ipsilateral | Both, but mostly ipsilateral |
Type of carcinoma | Ductal or lobular | Mostly ductal |
RR value of subsequent cancer | ||
All women | 4.2 | 4.3 |
No family history | 3.5 | 3.2 |
Positive family history | 8.4 | 9.7 |
Age 20–30 | 0.0 | 7.0 |
Age 31–45 | 2.7 | 4.5 |
Age 46–55 | 6.4 | 3.5 |
Age 56–65 | 0.0 | 6.5 |
Age >65 | 0.0 | 5.0 |
Absolute risk | 13 % over 16 years | 12 % over 16 years |
Although it is generally accepted that ADH and DCIS share the same genetic abnormalities and hence may represent a continuum of the same disease process (Lakhani et al. 1995; Chuaqui et al. 1997), the role of usual hyperplasia as a putative precursor of breast cancer is not universally accepted (Boecker et al. 2002).
10.2.5 Management of Patients with ADH in a Needle Core Biopsy
The prevalence of ADH in needle core biopsies varies in different institutions and has been reported as 9 % (Liberman et al. 1994), 4.5 % (Moore et al. 1994), 3.4 % (Menes et al. 2009) and 1.2 % (Deslauriers et al. 2012). Despite the lack of specific mammographic abnormality, the diagnosis of ADH in a needle core biopsy should lead to an excisional biopsy because associated DCIS or invasive carcinoma cannot be excluded. Several studies have documented the presence of high-grade lesions in the excision specimens of patients with the diagnosis of ADH on needle core biopsy, usually termed underestimation of a high-grade lesion. When Ely and colleagues (2001) correlated the results of 47 needle core biopsies reported as showing ADH with the excision specimens, they reported the following lesions: benign lesions without atypia, 14; ADH, 13; ALH, 3; DCIS, 15; and invasive carcinoma, 2. The higher the number of cores with multiple foci of ADH, the more advanced the lesion in the excision biopsy (Kohr et al. 2010). In some studies, cases previously reported as showing ADH in needle core biopsies yielded in situ or invasive carcinoma in up to 50 % of the cases (Liberman et al. 1994; Gadzala et al. 1997).
In a separate study, Renshaw et al. (2001) reviewed 95 cases that met the authors’ criteria of ADH. The subsequent resection specimens showed DCIS in 13 patients, ADH in 31, LCIS in six and benign proliferative lesions in 45. These authors claim that the low prevalence of carcinoma in the excision biopsies was due to stringent criteria applied to the diagnosis of ADH. These figures are not significantly different from those of Ely and colleagues (2001). Lack of ADH in the excision specimen has also been attributed to complete removal of the small foci of ADH in the needle core biopsies. Complete removal of small foci of DCIS or ADH is frequently reported with the use of a mammotome (Adrales et al. 2000). Sneige et al. (2003) reported complete removal of ADH using directional vacuum-assisted biopsies in 24 out of 42 cases when they compared the needle core biopsies with the excision specimens; DCIS was identified in three cases and the remaining 15 showed residual ADH. Assessing the number of units (ducts and lobules) involved by ADH can assist in predicting which patients would have residual or high-grade disease in excision specimens; the higher the number of TDLUs involved, the more the likelihood of yielding a higher-grade lesion in the excision specimen (Ely et al. 2001; Sneige et al. 2003). Because some foci of calcified ADH are completely removed during vacuum-assisted biopsies, some authors advocate avoiding excisional biopsies in these patients if the follow-up mammograms lack microcalcification and there is appropriate radiological–pathological correlation (Adrales et al. 2000; Sneige et al. 2003).
In a separate study, Eby and colleagues (2009) compared the yield of ADH when using a 9-gauge vacuum-assisted breast biopsy device and an 11-gauge device. The authors also compared the frequency of upgrade of ADH to higher-grade lesion in the excision specimen. ADH was diagnosed in 141 out of 991 biopsies (14.2 %). The frequency of ADH was 83/600 (13.8 %) for the 9-gauge device and 59/391 (14.8 %) for the 11-gauge device. The yield of carcinoma (in situ and invasive) was 16/74 (21.6 %) for the 9-gauge device and 10/49 (20.4 %) for the 11-gauge device. The results show that there was no difference between 9-gauge and 11-gauge device.
10.2.6 Management of ADH on the Margin in a Lumpectomy Specimen
The decision to excise a mammographic detected abnormality following the diagnosis of ADH in a needle core biopsy is universally accepted. However, there is no consensus among pathologists or surgeons on how to manage ADH at the margin of a lumpectomy performed for early-stage cancer. This is compounded by the intra- and interobserver variation which makes the diagnosis of ADH unreliable. Nizri et al. (2012) sent a survey to the members of the American Society of Breast Surgeons (ASBS) and received 477 responses; 377 of the respondents dedicated more than 50 % of their practice to breast surgery and 50 % were from academic cancer centres or dedicated breast centres. When asked how to manage the diagnosis of ADH within 1 mm of breast conserving surgical specimens, 61 % favoured no further surgery while 30 % recommended selective re-excision. Eighty percent of surgeons practising at cancer centres would recommend no further surgery while 30 % recommended selective re-excision and 0 % recommended routine re-excision when ADH involved the margin. In contrast, 54 % of surgeons in private practice would recommend no further excision, 40 % would selectively re-excise and 5 % would routinely excise. In a separate study in the UK, a questionnaire was sent to 200 breast surgeons requesting responses related to various aspects of margin in breast conserving surgery. Ninety one percent of the respondents indicated they would not alter their treatment management if ADH was present on the margin but both invasive and in situ carcinoma were 10 mm clear of the margin. The remaining 9 % of the surgeons would reconsider re-excision or perform mastectomy (Young et al. 2007). Ghofrani et al. (2006) also demonstrated that there was no consensus among pathologists when faced with the diagnosis of ADH on the margin. The authors distributed five problem scenarios to 300 pathologists for them to decide whether the images were DCIS or ADH and if the lesion was on the margin, would the pathologist recommend re-excision. One of the images consisted of unequivocal DCIS adjacent to duct partially filled with cribriform proliferation. Of 230 respondents, 56.5 % considered the partial cribriform proliferation within a duct adjacent to unequivocal DCIS as ADH and 37.7 % would recommend re-excision if this was on the margin. Of the 43.5 % who diagnosed the partially involved duct as DCIS, 28.0 % would recommend re-excision if it were on the margin.
When Baker et al. (2012) reviewed the literature on the subject of evaluating ADH in breast-conserving surgery, they only found very few studies on this subject. One study was from the Mount Sinai Medical Centre (Arora et al. 2008) and the authors’ objective was to determine the rate of residual pathology in patients who had re-excision for ADH involving the margin. The study was a retrospective review of 44 lumpectomy specimens with ADH involving the margins reported between 2000 and 2006. Twenty-four patients (55 %) had re-excision. Slides were reviewed to verify the diagnosis of ADH near the margin and the presence of residual disease on the re-excision specimen. Fifteen patients (63 %) had pure ADH, 7 (29 %) had ADH and DCIS and two (8 %) had invasive carcinoma. Of the 15 patients who had ADH, 6 (40 %) had residual pathology, thus: ADH (2), DCIS (2) and invasive carcinoma (2). This was a very small study but the authors concluded that further re-excision was recommended if ADH was present on the margin of a lumpectomy specimen. An earlier study by Goldstein et al. (1998) reported ipsilateral recurrent disease in 6 out of 94 patients treated with local excision followed by radiotherapy when DCIS and ADH were 2 mm from the margin. The follow-up for this study was only 26 months. Greene and colleagues (2006) reviewed 747 patients with biopsy proven ADH and 155 of these had pure ADH without associated premalignant or malignant breast disease. The excision biopsies of 68/155 (44 %) patients with pure ADH had negative margins and 7 (5 %) had positive margins and 80 (52 %) had no comment on the margin status. There was no re-excision of the patients with positive or close margins who were followed up for 0–119 months (mean 26 months). Seven patients presented with new findings at the site of their original excisional biopsy, six with benign lesions and one with invasive carcinoma. The authors concluded that clear margins at surgical excision for ADH did not affect the risk of developing subsequent malignancy. Based on the above publications, it is clear that there is no consensus on the management of ADH on the margin and further studies are required with long follow-up periods to establish the significance of ADH on the margin in a lumpectomy specimen.
Patients with ADH also benefit from tamoxifen as a chemopreventative strategy. In the Breast Cancer Prevention Trial (NSABP-P1), tamoxifen reduced the risk of breast cancer in 86 % of women with atypical hyperplasia (Fisher et al. 1998).
10.2.7 ADH as Risk Factor of Subsequent Malignancy
Because women with ADH can develop cancer in either breast, the proliferation used to be considered a marker for malignancy. However as previously alluded to ADH shares some genetic abnormalities with DCIS and invasive carcinoma which indicates a precursor lesion. Most of the information available on the risk of subsequent cancer associated with ADH is based largely on the work by Page and colleagues (1985). The authors identified 377 biopsies (3.6 %) with atypical epithelial hyperplasia from a total of 10,542 biopsies. The atypical proliferations consisted of 2.1 % ADH and 1.6 % atypical lobular hyperplasia (ALH). The women with the diagnosis of ADH and other benign breast disease were followed up for an average of 17 years (range 1.4–24.3 years). Eighteen women out of 150 with ADH subsequently developed invasive cancer, with ten of the cancers arising in the ipsilateral breast. Fourteen of the women developed cancer within 10 years of follow-up. The average age of women with ADH was 46 years (range 20–84 years), and for those who developed cancer the average age was 48 years (range 28–73 years). From these findings, the calculated RR of developing breast cancer in women with ADH was 4.4 times that of women without proliferative disease. The calculated absolute risk of women with ADH developing invasive cancer within 10–15 years of biopsy in the ipsilateral or contralateral breast was approximately 10 %. The presence of family history (mother, sister or daughter) increased the RR to 8.9 times that of the general population with an absolute risk of about 20 % at 15 years. In essence, the combination of ADH and family history doubles the risk for breast cancer to that in line with DCIS. DCIS is associated with 10–11 times the risk of subsequent invasive carcinoma (Rogers 1987). The combination of atypical hyperplasia and calcification increases the RR from 4.4 to 6.5.
Tavassoli and Norris (1990) reported similar observations when they followed up 82 patients with atypical intraductal hyperplasia from 1.4 to 27.8 years (median 12.4 years). Seven out of eight patients who developed invasive carcinoma did so within 17 years of follow-up. They calculated the RR of ADH for developing subsequent carcinoma to be 4.7. Six of the patients had ipsilateral cancer. Palli et al. (1991) reported an RR of 13 in women with ADH, but this was a relatively small study with a short follow-up period.
10.2.8 Follow-Up of Patients with ADH
If a higher risk lesion was excluded by excisional biopsy in patients with the diagnosis of ADH, Page (1992) advocated annual mammography of both breasts with the aim of detecting cancer at an early curable stage. Women with ADH who develop cancer during follow-up do so within 10–15 years (Dupont and Page 1985; Page and Dupont 1992; Tavassoli and Norris 1990). The risk of cancer declines after 10 years. In Institutions which utilise the Gail Model for risk assessment, Kiluk et al. (2007) divide the patients into low risk if Gail Model score is less than 1.67 and high risk if the score is more than 1.67. Patients with a Gail Model score of less than1.67 require routine screening of yearly clinical examination and yearly mammography. Women with a Gail Model score of more than 1.67 are followed by 6-monthly clinical examinations and yearly mammography with or without MRI. Genetic testing should be considered if there is a family history of breast cancer. The women should also be offered the opportunity to discuss the risk and benefits of chemoprevention using selective oestrogen receptor modulators (SERM) such as tamoxifen. Tamoxifen inhibits binding oestrogen to the oestrogen receptor. In the Breast Cancer Prevention Trial, the National Surgical Adjuvant Breast and Bowel Project (NSABP1) P-1 compared tamoxifen and placebo in over 1,300 women with a Gail Model score of ≥1.66 %. The study was halted due to a 49 % reduction in invasive breast cancer. More specifically, tamoxifen reduced the risk of breast cancer in 86 % of patients with atypical hyperplasia. The RR of adverse effects of tamoxifen such as endometrial cancer and thromboembolism were reported as recorded as 2.53 and 3.01, respectively. Prophylactic mastectomy should be considered only in patients with genetically proven susceptibility when regular mammographic follow-up is unacceptable (Simmons and Osborne 1999); the rationale being that mastectomy would remove more breasts than would necessarily develop cancer.
10.3 Lobular Neoplasia
10.3.1 The Concept of Lobular Neoplasia
Haagensen et al. (1978) introduced the term lobular neoplasia (LN) to avoid the use of the term carcinoma in an era when conservative management of breast cancer was uncommon. In this detailed clinico-pathological discussion, it is clear that lobular neoplasia was used for both ALH and LCIS, as the authors describe the early lesion with proliferation of neoplastic cells, which ‘eventually fill up the acini and obliterate their lumens’. There is evidence from follow-up studies that when properly classified, the risk associated with ALH is lower than that of LCIS (Dupont and Page 1985). Rosen (2001) believes that the term LN should be used to indicate a spectrum of lobular proliferations, which include mild atypia, atypical hyperplasia (ALH) and fully developed LCIS. Despite combining ALH and LCIS in the same category, the RRs for ALH and LCIS are four and 10–11 times that of the general population, respectively (Dupont and Page 1985; Page et al. 1991). The term lobular neoplasia is now included in the WHO classification of breast tumours (Tavassoli and Devilee 2003) and UK NHS Breast Screening Programme guidelines on report Breast Cancer (2005).
Mastracci et al. (2007) took a broader view on the classification of LN which the authors base on the extent of the disease within the terminal duct and lobular unit and within the breast in general. The authors classified lobular neoplasia in six categories:
(a)
Minimal atypical lobular hyperplasia when the lobular units are expanded by four to five cells across an acinus diameter
(b)
Atypical lobular hyperplasia when the TDLU is populated by lobular cells with a minimum number of eight cells across based on the study by Page and colleagues (1985)
(c)
ALH with ductal involvement by cells of ALH (DALH), usually termed pagetoid spread when of ALH undermine the normal ductal epithelium
(d)
LCIS when there is marked distortion and distension of more than 50 % of acini within a lobular unit
(e)
Invasive lobular carcinoma
The authors claim that it is important to distinguish minimal ALH from conventional ALH because the latter confers a higher risk of subsequent malignancy. Because of this confusion in terminology, Galimberti and co-authors (2013) suggest abandoning the terms DCIS and LCIS and replace them with ductal intraepithelial neoplasia (DIN) and lobular intraepithelial neoplasia (LIN) terms originally introduced by Tavassolli (1998, 2005). The authors claim the terms are confusing; DIN will eliminate the word carcinoma in lesions which do not metastasise, reduce risk of overtreatment and will be in line with other intraepithelial neoplasias of the cervix, vagina, vulva and prostate.
The cell type in ALH and LCIS is identical and the major difference between the two is that in ALH, 50 % of the lobules are partially filled by lobular neoplastic cells whereas in LCIS the lobules are completely filled (Page et al. 1991). In both symptomatic patients and the screening population, lobular neoplasia is usually an incidental finding in biopsies excised for another abnormality such as fibrocystic change, radial scar, fibroadenoma or invasive carcinoma.
10.3.2 Genetic Alterations in Lobular Neoplasia
Using the technique of comparative genomic hybridisation, Lu et al. (1998




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree