6 Erectile Dysfunction • Clinical Scenario 1 (Fig. 6–1) A 23-year-old male who has had erectile dysfunction (ED) for ~2 years. Patient states that his problem is difficulty in getting and maintaining an erection. The remainder of the medical history is noncontributory. • Clinical Scenario 2 (Fig. 6–2) A 75-year-old male who has had erectile dysfunction since radiation therapy for prostate cancer 3 years ago. Patient is hypertensive and is on oral medication. There is a question of heart disease, but no history of diabetes, smoking, Peyronie’s disease, or pelvic trauma. • Clinical Scenario 3 (Fig. 6–3) A 45-year-old male who has had difficulty in getting and maintaining an erection for the last 6 or 7 years. It has increased in severity recently. The patient has a history of prior alcohol and drug abuse and prior episode of prostatitis, which was treated with antibiotics. When he was younger, he did a considerable amount of bicycle riding and did develop transient numbness in the genital area. • Clinical Scenario 4 (Fig. 6–4) A 56-year-old male who has had erectile dysfunction progressing for several years. The patient denies hypertension, smoking, coronary artery disease, diabetes, Peyronie’s disease, pelvic trauma, or prostate cancer. • Clinical Scenario 5 (Fig. 6–5) A 71-year-old male who has had erectile dysfunction for 20 years. Patient has had two previous transurethral resections of the prostate. Patient has unstable angina. There is no history of hypertension, smoking, diabetes, Peyronie’s disease, pelvic trauma, or prostate cancer. • Clinical Scenario 6 (Fig. 6–6) A 51-year-old male who has had erectile dysfunction for ~5 years associated with Peyronie’s disease. The patient denies hypertension, smoking, coronary artery disease, diabetes, pelvic trauma, or prostate cancer. In July 1999, the First International Consultation on Erectile Dysfunction (ED) convened in Paris. It was cosponsored by the World Health Organization, International Consultation on Urological Diseases, and Société Internationale d’Urologie. ED was redefined as the consistent or recurrent inability to attain and/or maintain penile erection sufficient for sexual performance. A 3-month duration of ED is required to meet the criteria of consistency; the exceptions are cases of trauma or surgically induced ED. The term ED should not be used to describe penile curvatures, prolonged erection, painful erection, premature ejaculation, anorgasmia, or lack of desire.1 In 1995, it was estimated that there were over 152 million men worldwide who experienced ED; it is projected there will be −322 million with ED in 2025, an increase of nearly 170 million men. The largest projected increases will be in the developing world (i.e., Africa, Asia, and South America).2 In 1994, the Massachusetts Male Aging Study (MMAS) reported that the prevalence of minimal, moderate, and complete impotence was 52%. The prevalence of complete impotence tripled from 5 to 15% between subject ages 40 and 70 years.3 An update of the MMAS in 2000 indicated the incidence rate for erectile dysfunction was 25.9 cases per 1000 man-years [95% confidence interval (CI) 22.5 to 29.9]. The annual incidence rate increased with each decade of age and was 12.4 cases per 1000 man-years (95% CI 9.0 to 16.9), 29.8 (24.0 to 37.0), and 46.4 (36.9 to 58.4) for men 40 to 49, 50 to 59, and 60 to 69 years old, respectively. The age-adjusted risk of ED was higher for men with lower education, diabetes, heart disease, and hypertension. Population projections for men 40 to 69 years old are 17,781 new cases of ED in Massachusetts and 617,715 in the United States (white males only) annually.4 The penis is composed of three cylindrical structures: two dorsally located corpora cavernosa, and one ventrally located corpus spongiosum. The corpus spongiosum contains the anterior urethra. Distally, the corpus spongiosum expands to become the glans penis. The proximal ends of the corpora cavernosa, the crura, originate at the under-surface of the puboischial rami as two separate structures, but merge under the pubic arch and remain attached up to the glans. The septum between the two corpora cavernosa is incomplete in men. Blood supply to the corpus spongiosum and glans is through bilateral dorsal arteries (Fig. 6–1C
Differential Diagnosis
Prevalence
Anatomy of the Penis
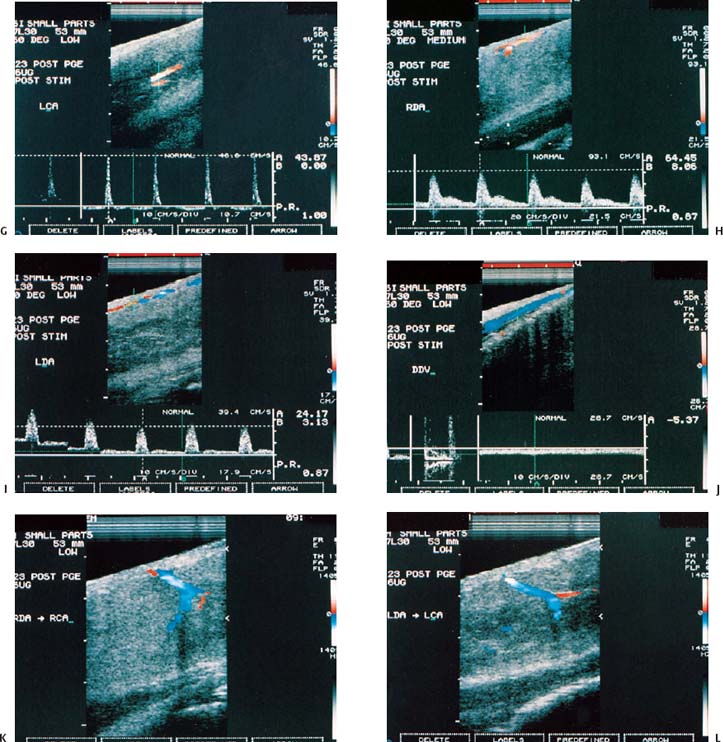
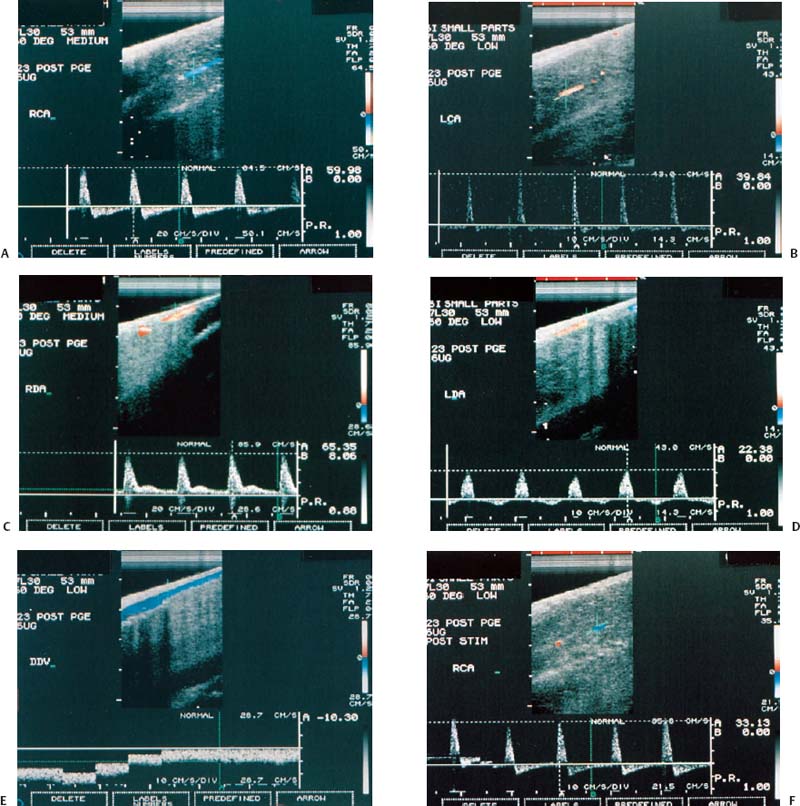
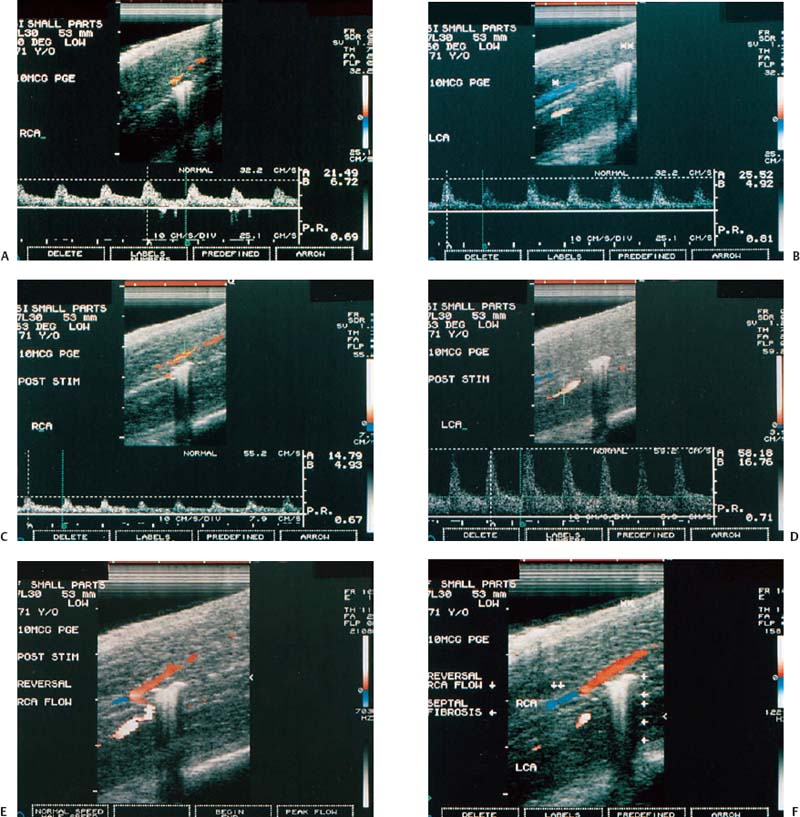
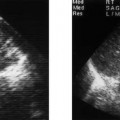
Figure 6–1 Clinical scenario 1. (A–E) Following intracavernosal injection of 6 µg of prostaglandin-E1 (Caverject), erectile response was 5/5. (A) RCA (PSV, 60 cm/s; EDV, 0 cm/s; RI, 1.0; reversal of diastolic flow). (B) LCA (PSV, 39.8 cm/s; EDV, 0 cm/s; RI, 1.0; reversal of diastolic flow). (C) RDA (PSV, 65.4 cm/s; EDV, 8.1 cm/s; RI, 0.88). (D) (PSV, 22.4 cm/s; EDV, 0 cm/s; RI, 1.0). (E) DDV (Peak velocity, 10.3 cm/s). (F–J) Following self-stimulation, erectile response was 5/5. (F) RCA (PSV, 33.1 cm/s; EDV, 0 cm/s; RI, 1.0; reversal of diastolic flow). (G) LCA (PSV, 43.9 cm/s; EDV, 0 cm/s; RI, 1.0; reversal of diastolic flow). (H) RDA (PSV, 64.5 cm/s; EDV, 8.1 cm/s; RI, 0.87). (I) LDA (PSV, 24.2 cm/s; EDV, 3.1 cm/s; RI, 0.87). (J) DDV (Peak velocity, 5.4 cm/s). (K) RDA to RCA collateral. (L) LDA to LCA collateral. Results: normal study. Diagnosis: Psychogenic erectile dysfunction. RCA, right cavernous artery; LCA, left cavernous artery; RDA, right dorsal artery; LDA, left dorsal artery; DDV, deep dorsal vein; PSV, peak systolic velocity; EDV, end-diastolic velocity; RI, resistive index.
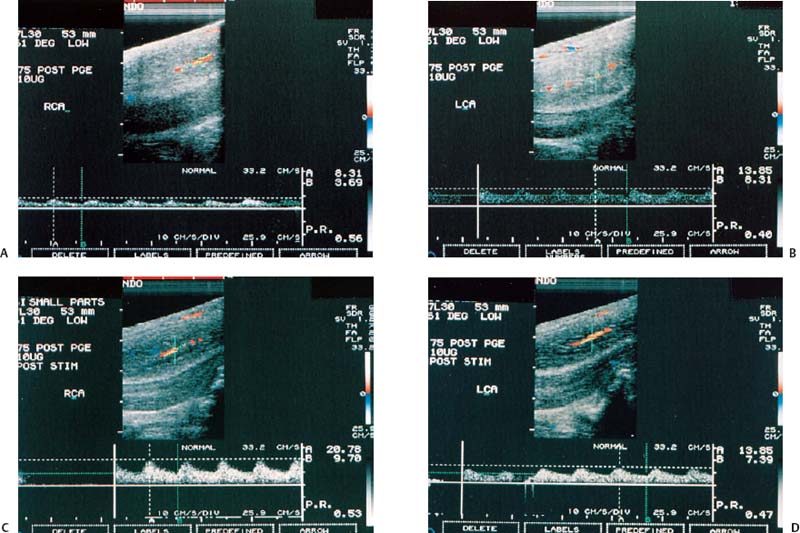
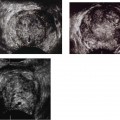
Figure 6–2 Clinical Scenario 2. (A,B) Following intracavernosal injection of 10 µg of prostaglandin-E1, (Caverject), erectile response was 1/5. (A) RCA (PSV, 8.3 cm/s; EDV, 3.7 cm/s; RI, 0.56). (B) LCA (PSV, 13.9 cm/s; EDV, 8.3 cm/s; RI, 0.40). (C,D) Following self-stimulation, erec-tile response was 1/5. (C) RCA (PSV, 20.8 cm/s; EDV, 9.7 cm/s; RI, 0.53). (D) LCA (PSV, 13.9 cm/s; EDV, 7.4 cm/s; RI, 0.47). Results: Bilateral cavernosal arterial insufficiency; cannot exclude cavernosal venous occlusive dysfunction. Diagnosis: Arteriogenic erectile dysfunction. RCA, right cavernous artery; LCA, left cavernous artery; PSV, peak systolic velocity; EDV, end-diastolic velocity; RI, resistive index.
The venous drainage from the corpora originates in tiny venules leading from the peripheral sinusoids immediately beneath the tunica albuginea. These venules travel in the trabeculae between the tunica and the peripheral sinusoids to form the subtunical venous plexus before exiting as the emissary veins. The emissary veins usually take an oblique course between the two layers of the tunica albuginea. Emissary veins draining the proximal corpora cavernosa join to form cavernous and crural veins. These veins join the periurethral veins from the urethral bulb to form the internal pudendal veins. The emissary veins from the more distal corporus cavernosum and spongiosum drain dorsally to the deep dorsal vein, laterally to the circumflex vein, and ventrally to the periurethral vein. Beginning at the coronal sulcus, the prominent deep dorsal vein (DDV) (Fig. 6–1E) constitutes the main venous drainage of the glans penis, corpus spongiosum, and distal two thirds of the corpora cavernosa. Usually one, but sometimes more than one, DDV runs upward behind the symphysis pubis to join the periprostatic venous plexus. Variations in the number, distribution, and termination of the venous systems are common.5
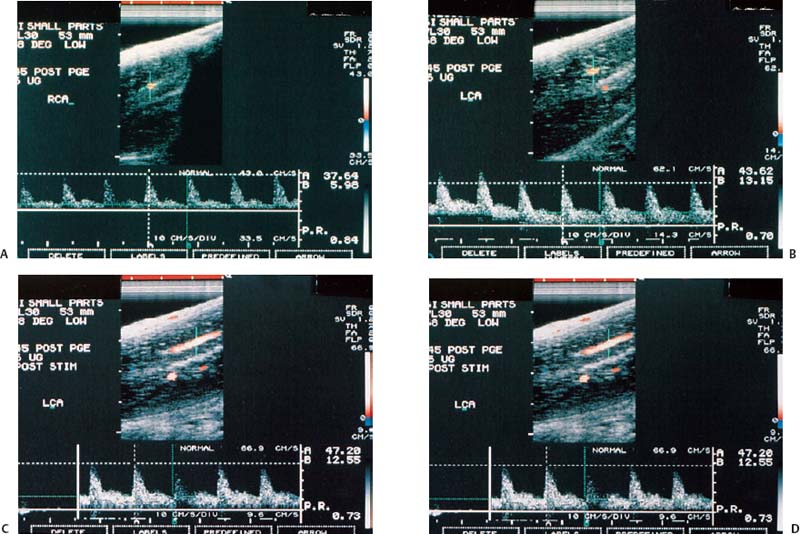
Figure 6–3 Clinical Scenario 3. (A,B) Following intracavernosal injection of 6 µg of prostaglandin-E1 (Caverject), erectile response was 2/5. (A) RCA (PSV, 37.6 cm/s; EDV, 6 cm/s; RI, 0.84). (B) LCA (PSV, 43.6 cm/s; EDV, 13.2 cm/s; RI, 0.70). (C,D) Following self-stimulation, erec-tile response was 3/5. (C) RCA (PSV, 38.2 cm/s; EDV, 7.8 cm/s; RI, 0.80). (D) LCA (PSV, 47.2 cm/s; EDV, 12.6 cm/s; RI, 0.73). Results: Cavernosal venous occlusive dysfunction. Diagnosis: venogenic erec-tile dysfunction. RCA, right cavernous artery; LCA, left cavernous artery; PSV, peak systolic velocity; EDV, end-diastolic velocity; RI, resistive index.
The innervation of the penis is both autonomic (sympathetic and parasympathetic) and somatic (sensory and motor). The parasympathetic (S2–S4) activity is responsible for tumescence and the sympathetic (T11–L2) activity causes detumescence.5
Physiology of Erection
The penile erectile tissue, specifically the cavernous smooth musculature and the smooth muscles of the arteriolar and arterial walls, plays a key role in the erectile process. In the flaccid state, these smooth muscles are chronically contracted by the sympathetic discharge, allowing only for a small amount of arterial flow for nutritional purposes.5 Sexual stimulation triggers the release of neurotransmitters from the cavernous nerve terminals. This results in relaxation of the smooth muscles and the following events: (1) dilatation of the arterioles and arteries with increased blood flow in both the diastolic and systolic phases; (2) trapping of the incoming blood by the expanding sinusoids; (3) compression of the subtunical venous plexuses between the tunica albuginea and the peripheral sinusoids, reducing the venous outflow; (4) stretching of the tunica to its capacity, which encloses the emissary veins between the inner circular and the outer longitudinal layers of the tunica and further decreases the venous outflow to a minimum; (5) an increase in intracavernous pressure (maintained at around 100 mm Hg), which raises the penis from the dependent position to the erect state (the full erection phase); and (6) a further pressure increase (to several hundred mm Hg) with contraction of the ischiocavernosus muscles (rigid erection phase).5
There are three types of erection: psychogenic, reflexogenic, and nocturnal.5 Psychogenic erection is the result of audiovisual stimulation or fantasy. Impulses from the brain modulate the spinal erection centers (T11–L2 and S2–S4) to activate the erectile process. There is an increased formation of nitric oxide (NO) by the nonadrenergic/noncholinergic neurons and endothelial cells, which stimulates the production of cyclic guanosine monophosphate (cGMP), resulting in the depletion of intracellular calcium and relaxation of smooth muscle resulting in erection. To return to the flaccid state, cGMP is broken down by phosphodiesterase type 5 (PDE5). Sildenafil (Viagra, Pfizer, New York, New York) is the first oral medication to inhibit PDE5 and prolong the effects of cGMP. It was also the first oral medication to receive Food and Drug Administration (FDA) approval. Currently, there are three oral medications on the market that inhibit PDE5 [sildenafil (Viagra); tadalafil (Cialis, Lilly ICOS, Indianapolis, Indiana); vardenafil (Levitra, Schering, Kenilworth, New Jersey)], which are used to treat ED.
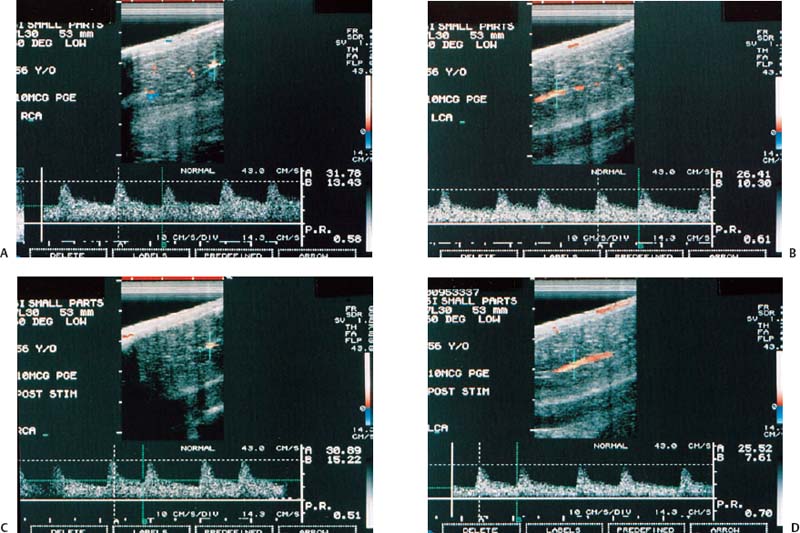
Figure 6–4 Clinical Scenario 4. (A,B) Following intracavernosal injection of 10 µg of prostaglandin-E1 (Caverject), erectile response was 2/5. (A) RCA (PSV, 31.8 cm/s; EDV, 13.4 cm/s; RI, 0.58). (B) LCA (PSV, 26.4 cm/s; EDV, 10.3 cm/s; RI, 0.61; irregular heart rhythm). (C,D) Following self-stimulation, erectile response was 2/5. (C) RCA (PSV, 30.9 cm/s; EDV, 15.2 cm/s; RI, 0,51). (D) LCA (PSV, 25.5 cm/s; EDV, 7.6 cm/s; RI, 0.70). Results: Mild bilateral cavernosal arterial insufficiency with cavernosal venous occlusive dysfunction. Diagnosis: venogenic erectile dysfunction with mild arteriogenic component and cardiac arrhythmia. RCA, right cavernous artery; LCA, left cavernous artery; PSV, peak systolic velocity; EDV, end-diastolic velocity; RI, resistive index.
Reflexogenic erection is produced by tactile stimuli to the genital organs. The impulses reach the spinal erection centers; some of them follow the ascending tract, resulting in sensory perception, whereas others activate the autonomic nuclei to send messages via the cavernous nerves to the penis to induce erection. This type of erection is preserved in patients with upper spinal cord injuries.5
Nocturnal erections occur mostly during rapid-eye-movement (REM) sleep. The mechanism that triggers REM sleep is located in the pontine reticular formation. During REM sleep, the cholinergic neurons in the lateral pontine tegmentum are activated, whereas the adrenergic neurons in the locus caeruleus and the serotonergic neurons in the midbrain raphe are silent. This differential activation may be responsible for the nocturnal erections during REM sleep.5
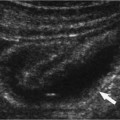
Figure 6–5 Clinical Scenario 5. (A,B) Following intracavernosal injection of 10 µg of prostaglandin-E1 (Caverject), erectile response was 1/5. (A) RCA (PSV, 21.5 cm/s; EDV, 6.7 cm/s; RI, 0.69). (B) LCA (PSV, 25.5 cm/s; EDV, 4.9 cm/s; RI, 0.81). (C,D) Following self-stimulation, erectile response was 3/5. (C) RCA (PSV, 14.8 cm/s; EDV, 4.9 cm/s; RI, 0.67). (D) LCA (PSV, 58.2 cm/s; EDV, 16.8 cm/s; RI, 0.71). (E) LCA to RCA collateral. (F) Reversal of flow in proximal RCA (double arrow) and septal fibrosis (single arrow) without Peyronie’s deviation. Results: Right cavernosal arterial insufficiency with cavernosal venous occlusive dysfunction. Diagnosis: Mixed arteriogenic and venogenic erectile dysfunction. RCA, right cavernous artery; LCA, left cavernous artery; PSV, peak systolic velocity; EDV, end-diastolic velocity; RI, resistive index.
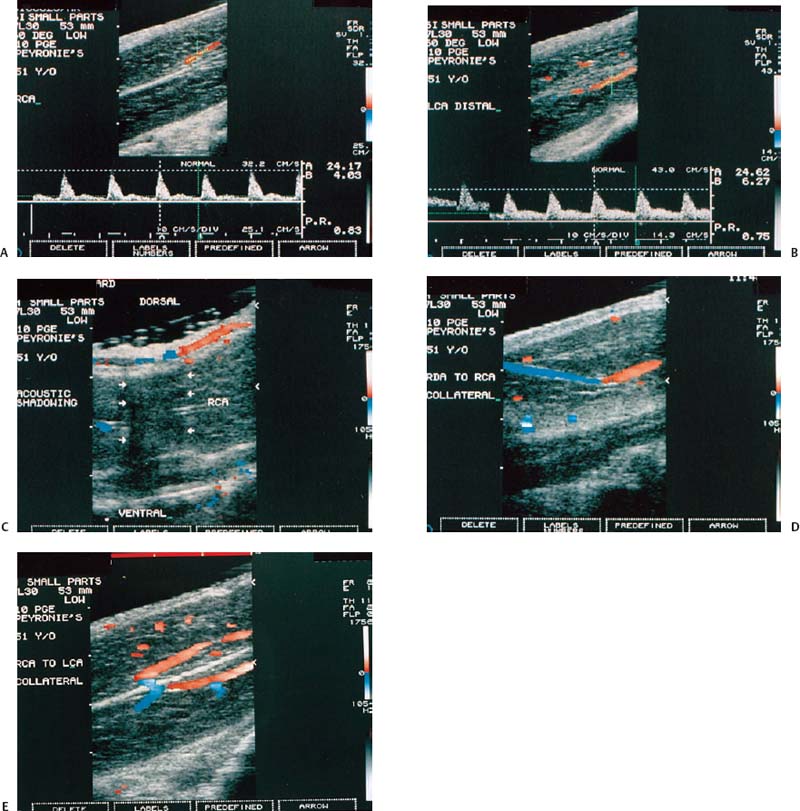
Figure 6–6 Clinical Scenario 6. (A,B) Following intracavernosal injection of 10 µg of prostaglandin-E1 (Caverject), erectile response was 3/5. No self-stimulation study was performed. (A) RCA (PSV, 24.2 cm/s; EDV, 4 cm/s; RI, 0.83). (B) LCA (PSV, 24.6 cm/s; EDV, 6.3 cm/s; RI, 0.75). (C) Distal dorsal plaque with acoustic shadowing (arrows) causing a dorsal penile curvature of ~45 degrees. (D) RDA to RCA collaterals. (E) RCA to LCA collaterals. Results: Penile curvature with dorsal plaque; mild bilateral cavernosal arterial insufficiency. Diagnosis: Peyronie’s disease. RCA, right cavernous artery; LCA, left cavernous artery; PSV, peak systolic velocity; EDV, end-diastolic velocity; RI, resistive index.
Classification of Erectile Dysfunction
Erectile dysfunction can be classified as psychogenic, neurogenic, endocrinologic, vasculogenic (arteriogenic, venogenic, mixed), or drug-induced.5
Psychogenic
Sexual behavior and penile erection are controlled by the hypothalamus, limbic system, and cerebral cortex. Therefore, stimulatory or inhibitory messages can be related to the spinal erection centers to facilitate or inhibit erection. Two possible mechanisms have been proposed to explain the inhibition of erection in psychogenic dysfunction: direct inhibition of the spinal erection center by the brain as an exaggeration of the normal suprasacral inhibition and excessive sympathetic outflow or elevated peripheral catecholamine levels, which may increase penile smooth muscle tone and thus prevent the relaxation necessary for erection.
Neurogenic
Because erection is a neurovascular event, any disease or dysfunction affecting the brain, spinal cord, cavernous and pudendal nerves, or receptors in the terminal arterioles and cavernous smooth muscles can induce dysfunction. Pathological processes involving the brain such as Parkinson’s disease, stroke, tumors, Alzheimer’s disease, and trauma can be associated with partial or complete erectile dysfunction. Spinal cord injuries, spina bifida, disk herniation, syringomyelia, tumor, and multiple sclerosis can affect the afferent or efferent neural pathways involving the spine and be associated with erectile dysfunction.
Because of the close relationship between the cavernous nerves and the pelvic organs, surgery on these organs is a frequent cause of impotence. The incidence of iatrogenic ED resulting from radical prostatectomy has ranged from 43 to 100%; abdominal perineal resection, 15 to 100%; and external sphincterotomy at the 3 and 9 o’clock positions, 2 to 49%. The introduction of nerve-sparing radical prostatectomy has dramatically reduced the incidence of impotence from nearly 100% to between 30 and 50%.
Alcoholism, vitamin deficiency, or diabetes may affect the cavernous nerve terminals, resulting in a deficiency of neurotransmitters. In diabetics, impairment of neurogenic and endothelium-dependent relaxation results in inadequate nitric oxide (NO) release.
Endocrinologic
The endocrine disorders that are associated with ED are androgen ablation therapy for prostate cancer, dysfunction of the hypothalamic–pituitary axis resulting in hypogonadism, prolactin-secreting pituitary tumors, hyperthyroidism, and hypothyroidism. Hyperprolactinemia, whether resulting from a pituitary adenoma or drugs, leads to both reproductive and sexual dysfunction. Symptoms may include loss of libido, ED, galactorrhea, gynecomastia, and infertility. Hyperprolactinemia is associated with low circulating levels of testosterone, which appear to be secondary to inhibition of gonadotropin-releasing hormone secretion by the elevated prolactin levels.
Hyperthyroidism is commonly associated with diminished libido, which may be due to the increased circulating estrogen levels and less often with erectile dysfunction. In hypothyroidism, low testosterone secretion and elevated prolactin levels contribute to ED.
Diabetes mellitus, although the most common endocrinologic disorder, causes ED through its vascular, neurological, endothelial, and psychogenic complications rather than through hormone deficiency per se.
Vasculogenic
Arteriogenic
Atherosclerotic or traumatic arterial occlusive disease of the hypogastric-cavernous-helicine arterial tree can decrease the perfusion pressure and arterial flow to the sinusoidal spaces, thus increasing the time needed to attain maximal erection and decreasing the rigidity of the erect penis. In the majority of patients with arteriogenic ED, the impaired penile perfusion is a component of the generalized atherosclerotic process. One study found that the incidence and age at onset of coronary disease and ED are parallel. Common risk factors associated with arterial insufficiency include hypertension, hyperlipidemia, cigarette smoking, diabetes mellitus, blunt perineal or pelvic trauma, and pelvic irradiation. Focal stenosis of the common penile or cavernous artery is most often seen in young patients who have sustained pelvic fracture or direct perineal trauma. Long-distance cycling is also a risk factor for vasculogenic and neurogenic ED.
Venogenic
Veno-occlusive dysfunction has been proposed as one of the most common causes of vasculogenic impotence. Veno-occlusive dysfunction may result from several possible pathophysiological processes: (1) the presence or development of large venous channels draining the corpora cavernosa, frequently seen in patients with primary ED; (2) degenerative changes (Peyronie’s disease, old age, and diabetes) or traumatic injury to the tunica albuginea (penile fracture), resulting in inadequate compression of the subtunical and emissary veins; (3) structural alterations in the fibroelastic components of the trabeculae, cavernous smooth muscle, and endothelium; (4) insufficient trabecular smooth muscle relaxation, causing inadequate sinusoidal expansion and insufficient compression of the subtunical venules, occurring in an anxious individual with excessive adrenergic tone or in a patient with inadequate neurotransmitter release from the parasympathetic nerves; and (5) acquired venous shunts (the result of operative correction of priapism), causing persistent glans-cavernosum or cavernosum-spongiosum shunting.
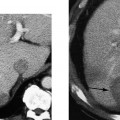
Peyronie’s disease is characterized by a lesion in the tunica albuginea of the corpora cavernosa (Fig. 6–6C). During erection, this lesion causes functional shortening and curvature of the involved aspect of the tunica. The lesion has commonly been called a plaque. Peyronie’s disease is seen in ~1% of white men. Although the disease has been reported in younger patients, there is a clear predominance between the ages of 45 and 60 years. The plaque is a focal scar that may result in susceptible individuals from an inflammatory response to microtrauma from bending and buckling of the erect penis during sexual intercourse. In the flaccid penis, the plaque is usually palpable as a nodule or thickening, often on the dorsal aspect of the penis.
Peyronie’s disease is also associated with the development of ED in some patients. It appears that in most cases, however, ED precedes the development of Peyronie’s disease.
Drug-Induced
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree