Trial/update
n
Risk
Groups (%)
Dose
Treatment modality
End point
Hormonal
therapy
Boston
Zietman et al. (2010)
393
Low
Intermediate
High
58
37
5
70.2 Gy
vs
79.2 Gy
3D
50.4 Gy/1.8 Gy +
proton boost
FFF 10 y
BF ASTRO
Phoenix
no
MD Anderson
Kuban et al. (2008)
301
Low
Intermediate
High
20
46
34
70 Gy
vs
78 Gy
3D
46 Gy/2.0 Gy +
boost
FFF 8 y.
BF ASTRO
no
Dutch
Al-Mamgani et al. (2011)
669
Low
Intermediate
High
18
27
55
68 Gy
vs
78 Gy
3D
50/Gy2.0 Gy +
boost
FFF 7 y.
BF ASTRO
Phoenix
6 months
–3 years
MRC
Dearnaley et al. (2007)
843
Low
Intermediate
High
24
32
44
64 Gy
vs
74 Gy
3D
64 Gy/2.0 Gy +
boost
FFF 5y.
BF nadir + 2 ng/ml
~6 months
RMH
Dearnaley et al. (2005)
126
Low
Intermediate
High
18
72
10
64 Gy
vs
74 Gy
3D
64 Gy/2.0 Gy +
boost
FFF 5y.
BF nadir + 2 ng/ml
~6 months
3 Influence of Radiation Therapy Dose on Progression-Free Survival
The long-term data from five randomised trials published between 2005 and 2010 confirmed the advantage of high dose radiation therapy for patients with localised adenocarcinoma of the prostate (Fig. 1).1
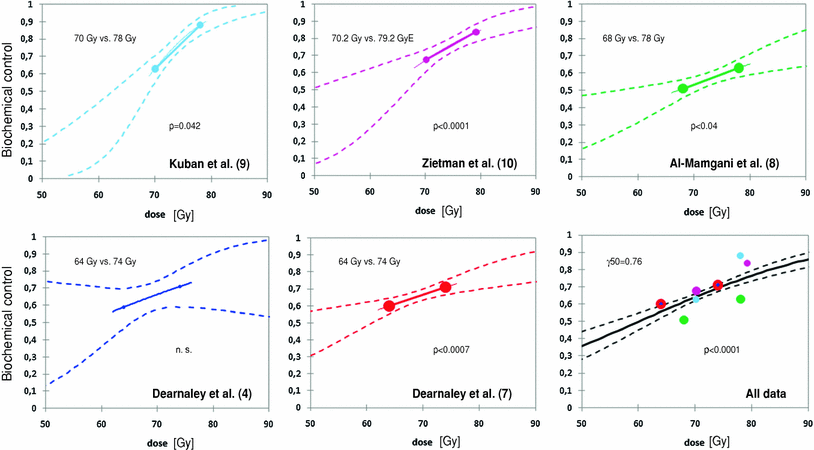

Fig. 1
Dose response curves in randomised trials on prostate cancer; broken lines 95 % confidence interval (patient numbers and dose were extracted from the respective publications, a correction for censored cases could not be carried out, thus the confidence intervals might be estimated a bit to narrow)
Dearnaley et al. (2005) published the results of a Royal Marsden NHS Trust and Institute of Cancer Research (RMH) phase III dose escalation pilot study. The total of 126 men with localised (T1–T3b) prostate cancer were randomised after an initial 3–6 month period of androgen suppression to deliver a dose of 64 Gy with or without a 10 Gy boost (64 and 74 Gy arms) between 1995 and 1997. The results showed that freedom from PSA failure was higher in the 74 Gy arm compared to the 64 Gy arm, but this did not reach conventional levels of statistical significance with 5-year actuarial control rates of 71 % in the 74 Gy arm vs. 59 % in the 64 Gy arm (p = 0.10). There was no difference in the time to restarting hormone therapy between the randomised groups (5-year actuarial rate 15–16 % in each group).
The following randomised Medical Research Council (MRC) RT01 trial with 843 men with localised prostate cancer (T1–T3a, PSA < 50 nag/mL), which were randomly assigned to escalated dose (74 Gy; n = 422) or standard-dose (64 Gy; n = 421) conformal radiotherapy was published by Dearnaley et al. (2007). After a median follow-up of 63 months, 5 year biochemical progression-free survival (bPFS) was a 71 % in the escalated and a 60 % in the standard group (p = 0.0007). In the subgroup analysis, no heterogeneity of effect on bPFS according to risk groups was found. Neither of these analyses showed that escalated dose treatment is more or less beneficial in either of these risk groups. The bPFS at 5 years for the standard and escalated groups were 79 and 85 % in the low-risk group, 70 and 79 % in the intermediate-risk group and 43 and 57 % in the high-risk group, respectively. No significant benefits were detected for clinical progression-free survival (p = 0.064), local control (p = 0.16), freedom from salvage androgen suppression (p = 0.12), and metastases-free survival (p = 0.21).
Al-Mamgani et al. (2008) presented the analysis of the Dutch dose escalation trial of radiotherapy for prostate cancer. A total of 669 patients with localised prostate cancer (T1–T4, PSA < 60 ng/mL) were randomly assigned 1997–2003 to receive either 68 or 78 Gy. After a median follow-up of 70 months, freedom from biochemical or clinical failure (FFF) using the ASTRO definition was significantly better in the 78-Gy arm than in the 68-Gy arm (7-year FFF rate, 54 % vs. 47 %, respectively; p = 0.04). The FFF using the Phoenix definition was also significantly better in the 78-Gy arm than in the 68-Gy arm (7-year FFF rate, 56 % vs. 45 %, respectively; p = 0.03). However, no difference was found between the high- and low-dose arms in freedom from clinical failure (70 % vs. 68 % at 7 years, respectively, p = 0.68).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree