Fig. 6.1
Several traditional and nontraditional factors are associated with the development of cardiac allograft vasculopathy
Evaluation
The clinical evaluation for CAV is challenging as the development and progression of disease is clinically silent due to the denervation of the transplanted heart. Even in the more advanced stages of disease, patients’ symptoms might be limited to dyspnea and exercise intolerance. Classic anginal symptoms might not be present making the clinical syndrome difficult to define [12, 13]. While clinical syndrome has proven to be elusive, the presence of even mild to moderate disease found on angiography has been associated with very poor outcomes with 17 % survival at 5 years [14]. Furthermore, if there is multi-vessel disease on angiography, the survival is abysmal at 13 % at 2 years [14]. Therefore, it’s imperative to have a low threshold to evaluate patients for ischemia early after the transplant as early aggressive medical management might alter the rate of progression of CAV.
Several imaging modalities might be helpful in establishing the diagnosis of disease early in its course. Noninvasive testing modalities including exercise electrocardiography, echocardiography, thallium scintigraphy, and exercise radionuclide ventriculography provide reasonable sensitivity and specificity in the diagnosis of CAV [15–19]. These imaging modalities do not yield the requisite accuracy in the diagnosis of CAV, however, as they cannot identify mild to moderate disease in the absence of hemodynamically significant lesions, that is, until late in the course of disease. Therefore, these tests lead to underdiagnosis in the early stages of disease and might be associated with undertreatment of CAV if used as the only diagnostic modality. Coronary multi-detector CT has a higher sensitivity due to visualization of the luminal and extraluminal characteristics of the vasculature and its role is yet to be defined in the transplanted patient cohort [20, 21].
Given the inherent limitations of the noninvasive methods and considering the morbidity and mortality associated with CAV in transplant recipients, surveillance coronary angiography is recommended (Class I, Level of Evidence C as per ISHLT) starting shortly after the transplant and annually thereafter. Routine angiography identifies focal and stenotic disease in 10 % of patients at 1 year and 50 % by 5 years [22]. Once mild disease is recognized by angiography, the likelihood of progression to severe disease is increased several fold [23]. While the gold standard in the diagnosis of coronary artery disease, coronary angiography has several limitations. Early in the course of CAV, there is no compromise in the luminal diameter of the vessels due to positive remodeling. Given that the angiogram essentially provides a luminographic assessment of the vasculature, its sensitivity is limited and leads to significant underdiagnosis of CAV [3, 24–26]. Furthermore, one of the strengths of angiography is its ability for the clinician to compare the abnormal segments with the normal segments—which is problematic in CAV as the disease process is diffuse and that there is no truly normal segment. Several studies have shown that coronary angiography alone has limited positive predictive value in the 40–50 % range [26, 27]. Using the angiogram, a TIMI frame count and myocardial blush grade may also help with identification of CAV; however, flow is usually normal in the early stages of disease and these techniques are of limited value [28].
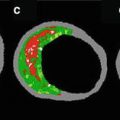
Intravascular ultrasound (IVUS) allows for high-resolution cross-sectional imaging of the vessel and provides not only accurate information about the vessel size but also the morphology of the vessel wall, thickening, and plaque composition. It is used widely during coronary interventions as it provides tomographic pictures of the wall as well as the lumen of the coronary artery. Given the information that IVUS can provide over and beyond coronary angiography, it has emerged as the optimal diagnostic tool for early detection of disease in native atherosclerotic disease. In the cardiac transplant patient cohort, IVUS is considered as a valuable adjunct in the early diagnosis of intimal thickening and arteriopathy (Fig. 6.2). Several studies have shown that where coronary angiography identifies 10–20 % of heart transplant patients to have CAV, IVUS detects intimal thickening in approximately 50 % of the patients at 1 year [3, 29]. Early diagnosis of allograft vasculopathy allows the clinician to be more aggressive about the early treatment of CAV.
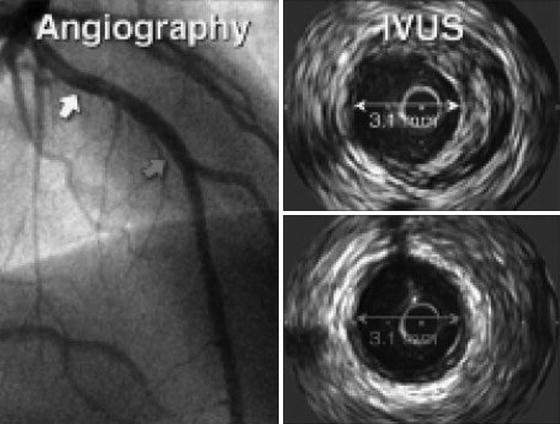

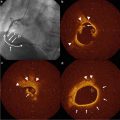
Fig. 6.2
Use of intravascular ultrasound leads to improved diagnostic accuracy as compared to coronary angiography. In this case, while coronary angiography shows similar luminal diameter in two areas of the vessel, IVUS shows that the more proximal segment has significant intimal hyperplasia whereas the distal segment has minimal disease. This case demonstrates the potential shortcomings of luminal angiographic assessment only
What Have We Learned About Cardiac Allograft Vasculopathy with IVUS?
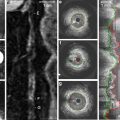
Serial IVUS studies have led to further understanding of the progression of CAV (Fig. 6.3). These studies have shown that coronary artery narrowing is influenced not only by progressive intimal hyperplasia but by the remodeling as well. A 5-year serial IVUS study has shown that most of the intimal thickening occurs during the first year after the transplantation. Intimal thickening is followed by an expansion of the external elastic membrane that preserves the lumen diameter as the vessel remodels. In later stages of disease, there is lumen loss as the negative remodeling occurs with constriction of the lumen area [30–32].
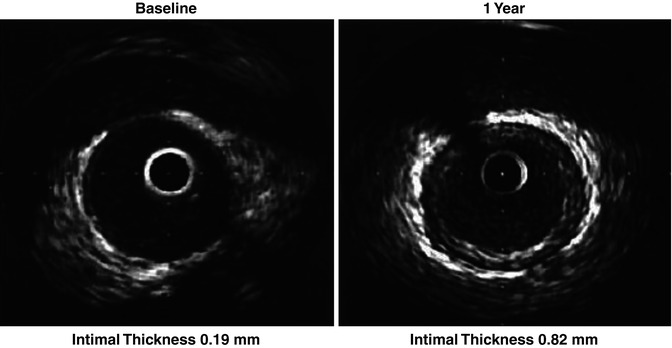

Fig. 6.3
Progression of disease can be monitored by serial IVUS imaging in patients with cardiac allograft vasculopathy as demonstrated below
Serial IVUS studies have been instrumental in determining the risk of progression as well. Traditionally, CAV is diagnosed when intimal thickness is >0.3 mm. Rapidly progressive CAV is defined as an increase of ≥0.5 mm in the first year of transplantation and has been associated with adverse outcomes correlating highly with angiographic development of disease [33–36]. Those with >0.6 mm intimal thickening even in the absence of angiographically visible disease are at significantly increased risk for cardiac events [35]. In contrast, those patients who receive donor-transmitted lesions do not have worse outcomes as compared to those associated with CAV—arguing for differences in pathophysiology and progression of these two distinct entities.
Given the usefulness of quantitative analysis of the intimal thickness, the Stanford scale has been developed to classify the lesions associated with CAV (Table 6.1). Case series have shown that in patients, where Stanford Grade 0–1 were found, CAV did not develop during follow-up whereas those with higher grade lesions are at higher risk of development of significant CAV [37].
Table 6.1
Maximal intimal thickness (MIT) and circumference involvement as defined for every measured segment using the Stanford classification
Stanford Grade | Definition |
|---|---|
Grade 0 | No evidence of an intimal layer |
Grade 1 | Intimal layer <0.3 mm involving <180° of vessel circumference |
Grade 2 | Intimal layer <0.3 mm involving ≥180° of circumference, or 0.3–0.5 mm with <180° of vessel circumference |
Grade 3 | Intimal layer 0.3–0.5 mm involving ≥180° of circumference, or 0.5–1.0 mm with <180° of vessel circumference |
Grade 4 | Intimal layer >0.5 mm involving ≥180° of vessel circumference, or >1 mm |
There are emerging data for the use of virtual histology-IVUS (VH-IVUS) where plaque composition can be studied. With this technology, plaque can be distinguished into four major components: necrotic core, calcium, fibrous, and fibrofatty. Studies have shown significant correlation between the VH-IVUS-derived plaque characteristics with the presence of diabetes mellitus, age of the donor, and male recipients. These studies have shown that in the first stages of intimal thickening, fibrous and fibrofatty components dominate, whereas necrotic core and calcification becomes more prominent at long term. The presence of necrotic core in males, older donors, and patients with diabetes mellitus especially with volume >2.01 mm3 is associated with the need for revascularization during follow-up. Furthermore, when plaque is characterized as inflammatory (necrotic core and dense calcium ≥30 %) and noninflammatory (necrotic core and dense calcium <30 %), those with inflammatory plaque were found to have higher risk of progression of CAV and early recurrent rejection. These findings are consistent with initial fibrous proliferation in the vasculature with CAV. Serial VH-IVUS studies have shown however that during long-term follow-up, the extent of necrotic core and calcification increase arguing for the dynamic process involved in the development of CAV [38–40].
Limitations and Safety of Intravascular Ultrasound
There are several limitations of IVUS imaging. Due to the size of the IVUS catheter, only proximal to mid epicardial vessels can be imaged leaving secondary and tertiary vessels unassessed. Furthermore, usual practice is to image only one coronary artery (usually the LAD) which further diminishes the sensitivity of the IVUS assessment. In a study, CAV was diagnosed in 58 % of patients where all 3 vessels were imaged at 1 year as opposed to 27 % seen in single-vessel imaging [41].
Safety of IVUS interrogation of the coronary tree has been well established. The rate of transient spasm varies from 1 to 3 % with vessel dissection or closure in 0.5 % of the cases. There had been some concern whether repeated IVUS assessment would accelerate CAV by causing endothelial injury; however, a serial longitudinal study has shown that IVUS is safe and does not contribute to progression of disease [42].
How Can the Information be Used in Patient Management?
Early diagnosis of CAV is of paramount value as adjustments in antirejection regimen and management of atherosclerotic risk factors might be organ- and life-saving. Several medications have been associated with improved outcomes when utilized in patients with CAV.
The use of rapamycin (sirolimus) in the immunomodulatory regimen has been associated with slowing the progression of CAV in several case series and is now widely accepted as the medication of choice in patients with CAV [43, 44]. Another immunosuppressive agent, mycophenolate mofetil (MMF) when compared to azathioprine has also been shown to reduce progression of intimal thickening [45]. Both rapamycin and MMF exert their action by inhibiting growth and activation of B and T cells, with proposed effect of slowing the degree of inflammation in the vessel wall leading to retardation of progression of CAV.
Several other drug categories have been associated with improved outcomes. Statins, via their pleiotropic effects, have been associated with slower progression of CAV [46, 47]. There are also data suggesting that combination therapy of angiotensin-converting enzyme (ACE) inhibitors and calcium channel blockers can attenuate the process of CAV [48, 49]. Interestingly, the treatment of CMV infection with ganciclovir can also slow the progression of CAV most likely by protecting the endothelium; which are otherwise targets for CMV infection [50]. Further therapeutic options for CAV include mechanical treatment of the coronary stenosis (percutaneous intervention) and coronary artery bypass grafting (CABG). Drug-eluting stents have been associated with lower restenosis rates as compared to bare metal stents in CAV consistent with treatment effects found with atherosclerotic disease [51]. Outcomes described with CABG have generally been poor, however, with high perioperative and 1-year mortality rates [52, 53].
Importantly, when assessing the impact of above therapies on the coronary vasculature in patients with CAV, IVUS has played an instrumental role in determining the progression of disease during longitudinal follow-up. This imaging modality has been used as the diagnostic outcome imaging of choice in assessing the effects of therapy.
When severe CAV is present and revascularization options are exhausted, retransplantation is the only effective therapy with survival rates in the 70–80 % range at 12–24 months [54]. Given the scarcity of donor organs however, retransplantation in this setting needs to be carefully assessed.
Conclusions
CAV, once present, is a major source of morbidity and mortality. Several treatment options are present, yet outcomes are poor unless the disease is detected early and aggressively treated. The use of highly sensitive techniques such as IVUS has enabled early diagnosis of disease with early aggressive treatment leading to improved outcomes.
References
1.
Taylor DO, Stehlik J, Edwards LB, et al. Registry of the International Society for Heart and Lung Transplantation: Twenty-sixth Official Adult Heart Transplant Report-2009. J Heart Lung Transplant. 2009;28:1007–22.PubMedCrossRef
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree