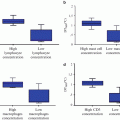
Fig. 3.1
Relationship between in-stent loss (ISL) and binary angiographic restenosis (Adapted from Brener SJ, Prasad AJ, Khan Z, Sacchi TJ. The relationship between late lumen loss and restenosis among various drug-eluting stents: a systematic review and meta-regression analysis of randomized clinical trials. Atherosclerosis. 2011; 214(1):158–62. With permission from Elsevier)
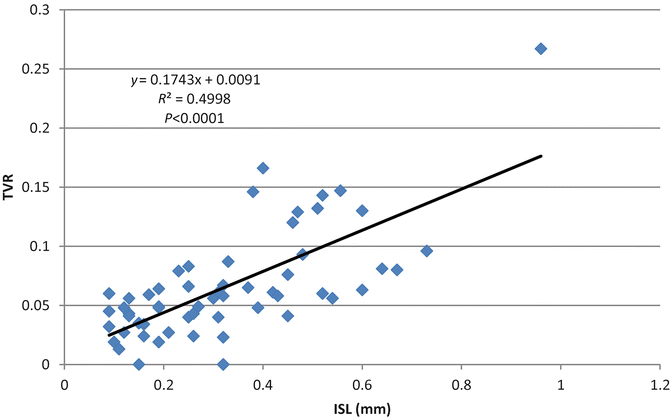
Fig. 3.2
Relationship between in-stent loss (ISL) and target vessel revascularization (TVR) (Adapted from Brener SJ, Prasad AJ, Khan Z, Sacchi TJ. The relationship between late lumen loss and restenosis among various drug-eluting stents: a systematic review and meta-regression analysis of randomized clinical trials. Atherosclerosis. 2011;214(1):158–62. With permission from Elsevier)
Table 3.1
Relationship between late lumen loss and angiographic or clinical restenosis
Drug | ISLLL (mm) | BAR (%) | TVR (%) |
|---|---|---|---|
SES | 0.17 | 8.0 | 5.7 |
PES | 0.40 | 13.1 | 8.4 |
EES | 0.15 | 4.7 | 3.5 |
ZES | 0.56 | 12.0 | 6.8 |
Other platforms | 0.44 | 15.1 | 11.3 |
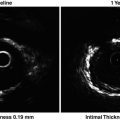
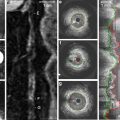
Beyond measurements of lumen size and evaluation of restenosis, QCA provides important insight into the process of reperfusion in patients with ST-elevation myocardial infarction (STEMI). Grading of flow in the infarct-related artery (IRA) after treatment with fibrinolytic therapy or with primary PCI provides critical prognostic information regarding survival. Using the TIMI classification [24], it was shown convincingly that patients with TIMI 3 flow in the IRA have better survival than those with lesser grades. In an analysis of two large STEMI trials with over 5,000 patients, final TIMI 3 flow increased survival at 1 year threefold, compared with TIMI 0–2 (HR = 3.67 [2.45, 5.48], P < 0.001) [25]. Because TIMI flow classification is semiquantitative, a fully quantitative evaluation of flow—corrected TIMI frame count (cTFC)—was developed by Gibson et al. [26]. They showed that flow was disturbed not only in IRA, but also in non-culprit arteries, compared with normal arteries in patients without STEMI (39.2 ± 20.0 vs. 25.5 ± 9.8 vs. 21.0 ± 3.1, P < 0.001), reflecting the heightened platelet reactivity and vascular tone in the former group. The same group extended these observations in patients treated with fibrinolytic therapy for STEMI and showed that cTFC was an independent predictor of survival at 2 years (P = 0.01), after adjusting for important baseline characteristics and revascularization [27].
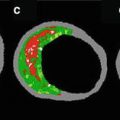
Even more important than epicardial flow is the tissue myocardial perfusion after STEMI. Ito et al. were among the first to demonstrate that nearly 40 % of patients with successful reperfusion of the IRA have no-reflow at the myocyte level, using myocardial contrast echocardiography. Patients with no-reflow had adverse ventricular remodeling, more heart failure, and other adverse outcomes [28]. Thus, accurate evaluation of myocardial reperfusion after primary PCI may help the operator tailor therapy, in the angiography suite and after the procedure, to the risk for adverse events. Myocardial reperfusion, or “blush score,” can be assessed using two paradigms. The dynamic method (TIMI myocardial perfusion grade—TMPG) assesses the entry and exit of contrast from the myocardium distal to initial IRA lesion [29]. The densitometric method (myocardial blush grade—MBG) compares the density of contrast opacification of the IRA territory to a reference territory [30]. Assessment of MBG can be computerized [31]. Both scales use grades from 0 to 3 and grades 2 or 3 indicate an open microcirculation [32]. In the HORIZONS AMI (Harmonizing Outcomes with Revascularization and Stents in AMI), myocardial reperfusion was assessed independently by both methods. Both TMPG 2 or 3 (HR = 0.53 [0.38, 0.73], P < 0.0001) and MBG 2 or 3 (HR = 0.54 [0.40, 0.75], P < 0.0001) were significant independent predictor survival at 3 years, even after adjusting for TIMI flow grade in IRA [33].
The principal limitation of QCA is its insensitivity to the functional significance of coronary stenoses. Recent advances in CT imaging promise to resolve this deficiency [34].
In summary, QCA plays a critical role in assessing the efficacy of intravascular devices and procedures. There is a robust correlation between measures of lumen loss and angiographic and clinical restenosis, which lead to TVR. QCA can reliably differentiate between the antiproliferative effects of various DES platforms and serves as an excellent surrogate endpoint for stent-vs.-stent comparisons. QCA-derived parameters of reperfusion are powerful predictors of survival and freedom from adverse events after STEMI.
Conclusions
Systematic and consistent evaluation of angiographic parameters has contributed significantly to improvements in CAD therapy, both for pharmacological interventions and for intravascular devices and procedures. Accurate measurement of changes in lumen and plaque size has highlighted the important dissociation between atherosclerosis regression and prevention of ischemic cardiac events. Changes in lumen size and even in plaque volume are small, yet they are associated with an important reduction in clinical events, suggesting that plaque modification and stabilization is more important than the change in size, particularly in earlier stages of the disease. Technologies geared at assessing plaque composition and vulnerability are much more critical to this field than QCA.
In contrast, the development and approval of intravascular devices is critically linked to QCA. Surrogate endpoints of most clinical trials incorporate angiographic parameters. QCA has proven able to differentiate between devices with reliability and accuracy. There is robust correlation between angiographic endpoints of lumen size and reperfusion and clinical endpoints.
References
1.
3.
Libby P, Ridker PM, Maseri A. Inflammation and Atherosclerosis. Circulation. 2002;105(9):1135–43.PubMedCrossRef
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree