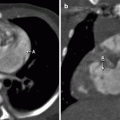
Fig. 7.1
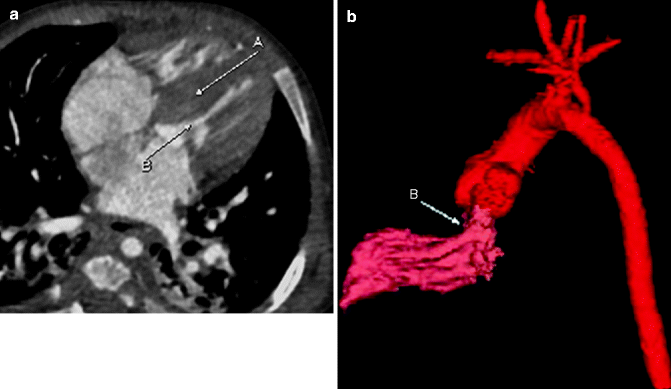
Sagittal maximum-intensity projection (MIP; a) and lateral projection color-coded three-dimensional (3D; b) images from a cardiac CTA demonstrating a normal three-vessel aortic arch. The sinus of Valsalva (E) typically bulges, with immediate narrowing at the sinotubular junction (F). The first vessel of a left aortic arch is the right brachiocephalic artery (A), which divides further into right subclavian (G) and right common carotid (H) arteries. The second vessel off the arch is the left common carotid artery (B) and the last is the left subclavian artery (C). Notice the typical ductus bump (D), which is seen along the undersurface of the descending aortic arch
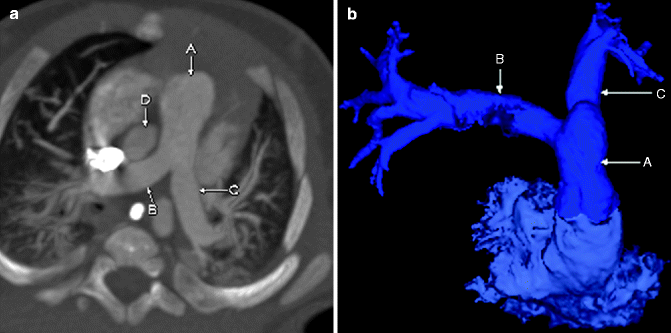
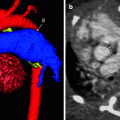
Fig. 7.2
Axial MIP (a) and 3D color-coded (b) images from a cardiac CTA. The main (A), right (B), and left (C) pulmonary arteries are shown. Notice the normal position of the ascending aorta (D) posterior to and to the right of the pulmonary outflow tract
PDA
PDA is the persistence of the normal prenatal vascular connection between the pulmonary artery and proximal descending aorta or brachiocephalic artery. In utero, the ductus arteriosus functions as a right-to-left shunt to bypass the pulmonary vasculature. After birth, it becomes a left-to-right shunt because of the higher pressures in the aorta relative to those in the pulmonary system. A PDA is a type of acyanotic heart disease with increased pulmonary vasculature, cardiomegaly, and a prominent “ductus bump.” A longstanding PDA may cause pulmonary hypertension, flow reversal, and cyanosis (Eisenmenger’s physiology). When it closes, a PDA forms a ligamentum arteriosum, which often calcifies.
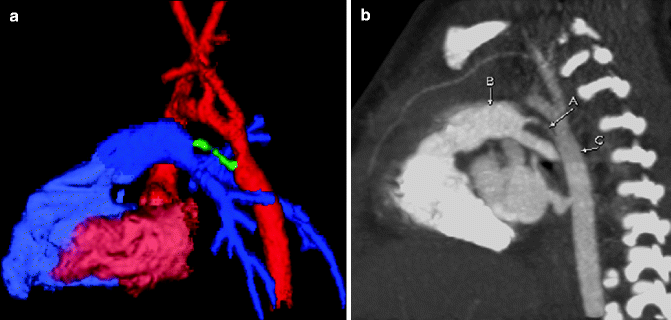

Fig. 7.3
Color-coded 3D (a) and sagittal MIP (b) images from a cardiac CT showing a small PDA (green, A) connecting the pulmonary artery (blue, B) to the proximal descending aorta (red, C)
PDA from the Left Brachiocephalic Artery
A PDA may arise from the aorta or left brachiocephalic/left subclavian artery. With a right arch and an aberrant subclavian artery, the PDA may complete a vascular ring when it arises from an aberrant left subclavian artery.
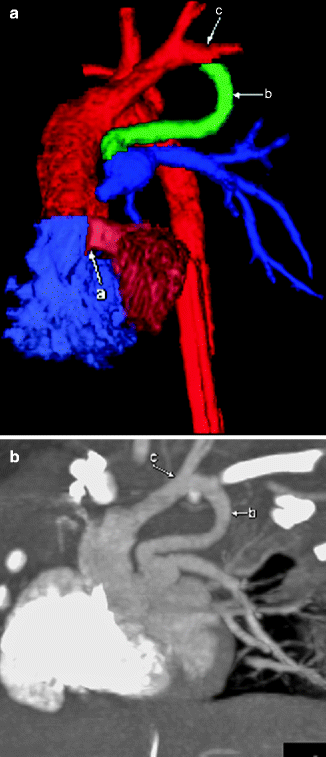

Fig. 7.4
Color-coded 3D (a) and sagittal MIP (b) images from a cardiac CT showing a PDA (b) attached to the left subclavian artery (c) in a patient with a right aortic arch. A ventricular septal defect (VSD; a) also is shown on the 3D image
PDA from the Descending Aortic Arch
A PDA often is necessary for survival, especially if there is obstruction to aortic flow, as seen in severe aortic stenosis, coarctation of the aorta, interruption of the aortic arch, and hypoplastic left ventricle. The PDA may be stented (hybrid procedure) in some patients with hypoplastic left ventricle.
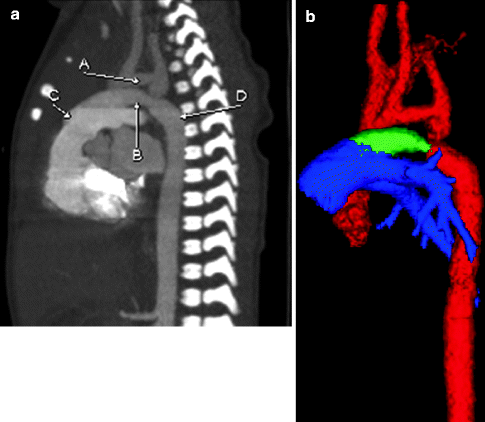

Fig. 7.5
Sagittal MIP (a) and color-coded 3D (b) images from a cardiac CT scan showing a PDA (B, green) connecting the descending aortic arch (D, red) and the pulmonary artery (C, blue). A hypoplastic aortic arch (A, red) also is shown
Bilateral PDA
Rarely, a PDA may be separate and bilateral, arising from the left brachiocephalic artery and aorta. In these cases, the patient typically has complex congenital heart disease with several other anomalies.
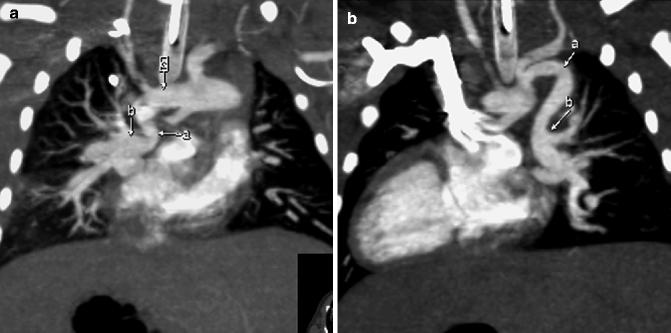
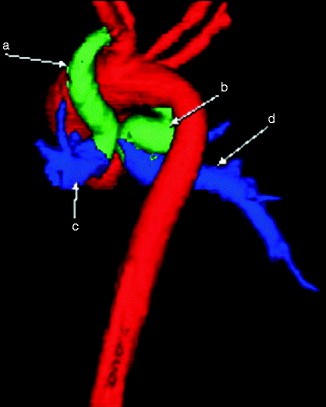

Fig. 7.6
(a) MIP image from a cardiac CT demonstrating a PDA (a) extending from the aortic arch (c) to give rise to the right pulmonary artery (b). (b) Coronal MIP image showing a second, tortuous PDA (a) from the descending aortic arch, giving rise to the left pulmonary artery (b)

Fig. 7.7
Color-coded 3D image from a cardiac CT scan showing bilateral PDAs (a and b) giving rise to the right (d) and left (c) pulmonary arteries
Aortic Atresia
Aortic atresia is part of the hypoplastic left heart syndrome and involves hypoplasia/atresia of the ascending aorta, aortic valve, left ventricle, and mitral valve. Patients depend on a PDA for survival; death occurs within days (as the PDA closes) if the condition is untreated. Patients have cyanosis, with chest radiography demonstrating cardiomegaly and pulmonary venous congestion. Echocardiography, CT, MRI, and angiography may be used for treatment planning. A variety of surgical options exist.
In aortic atresia, the coronary arteries receive retrograde blood flow from the PDA up to the aortic arch and then down the coronary arteries via a hypoplastic ascending aorta. The brain and upper extremities also depend on the retrograde flow from the PDA.
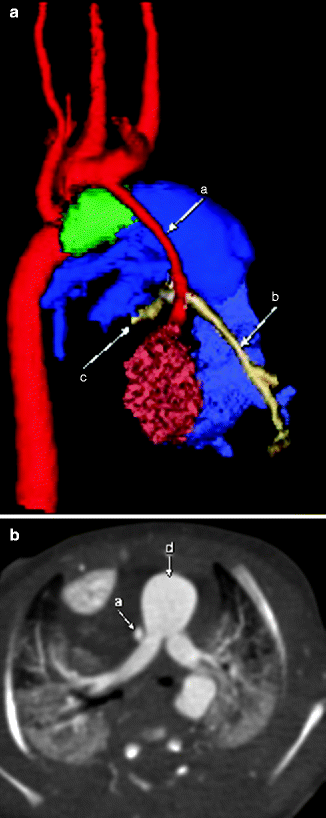

Fig. 7.8
3D color-coded (a) and axial (b) images demonstrating hypoplasia of the ascending aorta (a). The main pulmonary artery (d, blue) provides blood flow through a PDA (green), allowing retrograde flow to the great vessels coming off the arch (red) and the coronary arteries (tan, b and c)
Aortic Stenosis
Aortic stenosis may be valvular, subvalvular, or supravalvular (Williams syndrome). In neonates/infants, chest radiography is normal or shows mild cardiomegaly and pulmonary edema. In children/adolescents, chest radiography is often normal, even if the aortic stenosis is severe. Patients with aortic stenosis have thickening with fusion of the aortic valve leaflets, poststenotic dilatation of the ascending aorta, a systolic flow jet into the ascending aorta, and left ventricular hypertrophy. MRI and echocardiography may be used to assess the transvalvular pressure gradient, the regurgitant fraction, and ventricular function.
Valvular Aortic Stenosis
Valvular aortic stenosis, reported in 1–2 % of the population, most commonly is the result of a bicuspid aortic valve, although bicuspid aortic valves do not always cause significant stenosis. It is more common in males and has a familial predilection.
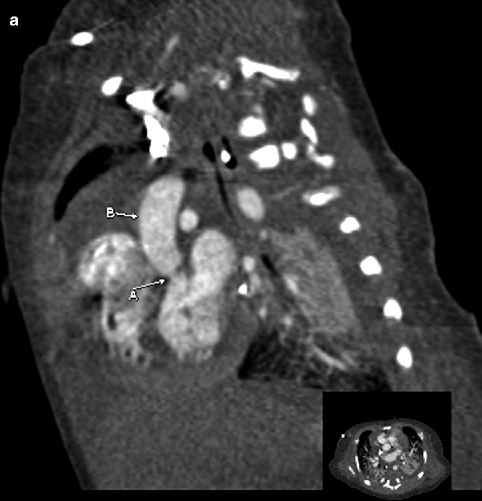
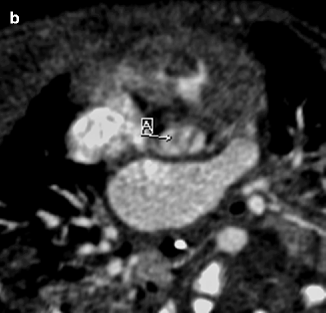


Fig. 7.9
Sagittal (a) and axial (b) contrast-enhanced cardiac CT images demonstrating narrowing of the aorta at the level of the aortic valve (A) with thickening of the bicuspid valve leaflets (A). Poststenotic dilatation of the ascending aorta (B) also is seen
Supravalvular Aortic Stenosis (Williams Syndrome)
Supravalvular aortic stenosis occurs above the aortic valve, giving the proximal aorta an “hourglass” appearance. It is associated with stenosis of other vessels, such as the pulmonary and coronary arteries and descending abdominal aorta. Children with this disease may have elf-like facial features and mental retardation.
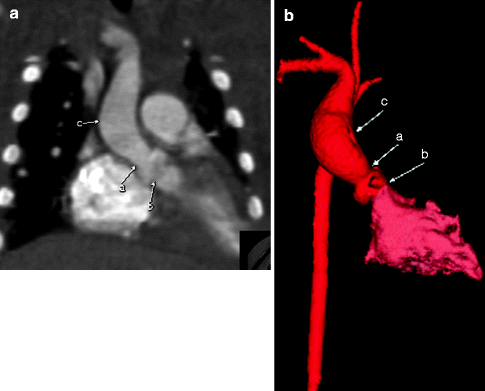
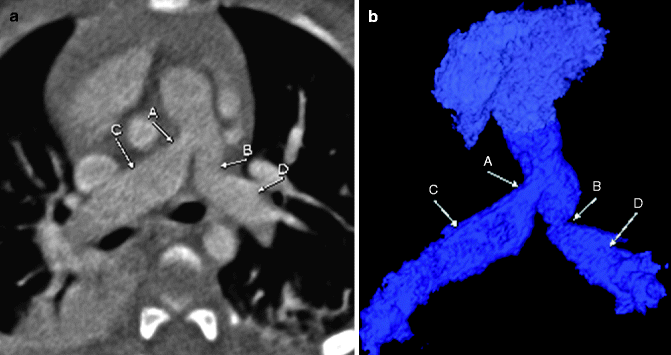

Fig. 7.10
Coronal MIP (a) and color-coded 3D (b) images from a cardiac CT scan in a patient with Williams syndrome. The images show supravalvar aortic stenosis (a) with poststenotic dilatation (c) of the aorta. Notice that the subvalvular region is of normal caliber (b). The contours of the ascending aorta often are described as hourglass shaped

Fig. 7.11
Axial (a) and color-coded 3D (b) images in a patient with known Williams syndrome. The images demonstrate stenosis of the proximal left (B) and right (A) pulmonary arteries with associated poststenotic dilatation (C and D). Pulmonic stenosis is a common finding in patients with Williams syndrome
Hypertrophic Cardiomyopathy with Subaortic Stenosis
Hypertrophic cardiomyopathy with subaortic stenosis, the most common form of cardiomyopathy in children, is marked by thickening of the interventricular septum, causing left ventricular outflow tract obstruction (LVOT). The etiology may be idiopathic. Infants of diabetic mothers also may develop significant hypertrophy of the interventricular septum and may have subvalvular stenosis.