Scanner
2nd gen. DSCT
Myocardial coverage
7.3 cm
Scan type
Shuttle mode
Scan duration
30 s
Contrast timing
Test bolus −4 s
Tube current and tube voltage
200 mAs, 100 kV
Contrast application
50 cc, 370–400 mg Iodine, 5 ml/s
Stressing agent
Adenosine, 140 μg/kg BW
Temporal resolution
<63 bpm: every heartbeat >63 bmp: every second heart beat
e.g., in a HR of 100 bpm: 12 acq. In 30 s, temporal resolution = 2.5 s
Radiation dose
~10 mSv
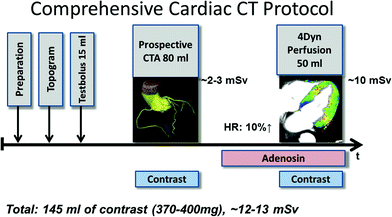
Fig. 1
A comprehensive cardiac CT protocol in patients with suspected coronary artery disease (CAD) could comprise a morphologic CT angiography of the coronary arteries and a dynamic perfusion scan of the myocardium. According to published data, the combination of CTA plus CT perfusion could increase the diagnostic accuracy in the detection of hemodynamically significant coronary artery stenosis, namely reducing the number of false-positive findings by CTA. Today, the typical total amount of contrast volume and radiation dose for such a protocol amounts to ~145 ml of contrast agent and 12–13 mSv-equivalent dose, respectively. In the future, technical improvements of the acquisition techniques will help to further reduce these values
Overall, a perfusion sequence on MDCT is comparable to an ECG-triggered test bolus technique. To obtain a reasonable signal-time curve with a modest amount of contrast agent, high speed injections with a saline flush technique are preferable to acquire good quality signal-time curves (e.g., ∼40–50 ml of contrast agent injected at a flow rate of about 5–8 ml/s). While all previous efforts in CT MPI were typically region-of-interest (ROI) based, i.e., measuring the time-density curves in a small portion of the myocardium defined by a ROI, recent studies have extended these principles into quantitative three-dimensional (3D) imaging techniques. It was shown that regional MBF can be derived in patients in combination with CT-based morphological assessment of CAD, i.e., CTCA (Bamberg et al. 2010, 2011; Wang et al. 2012).
There are many technical aspects that need further optimization in cardiac CT perfusion imaging. Even if modern techniques for reduction of radiation exposure are applied, typical study protocols employed for dynamic 3D imaging of the myocardium will still result in a comparably high total radiation exposure of an approximately 10–13 mSv-equivalent radiation dose (including the perfusion imaging and a prospective CTCA). On the other hand, this radiation exposure is similar to that of established retrospective CTCA examinations on 64-slice CT (with a reported median of 12 mSv in a large multicenter study) (Hausleiter et al. 2009) and comparable to or lower than nuclear perfusion imaging, the clinical standard for the assessment of myocardial perfusion (radiation exposure for stress-only 99 m technetium [Tc] sestamibi is approximately 10.0 mSv, but that for 2-day 99 mTc sestamibi or 201Tl is 20.0 mSv or higher) (Einstein et al. 2007).
Further technical research will be necessary in CT MPI, to develop strategies to reduce radiation exposure and determine the impact of heart rate and different injection protocols on image quality, diagnostic accuracy, and radiation exposure. Independent of the technical acquisition protocol used (single-source dynamic, single-source static, and dual-source/dual-energy static), the protocol includes the administration of adenosine as a stressing agent to better depict myocardial perfusion defects due to the induced “steal-effect” caused by the vasodilatation. Adenosine is a short-lasting vasodilator, and continuous ECG monitoring is mandatory. Contraindications for administration of adenosine include Wolff-Parkinson-White (WPW) syndrome, atrio-ventricular (AV) block, and sick-sinus syndrome as well as asthma. Finally, besides the development of dose-reduction algorithms and optimized contrast agent injection protocols for dynamic MPI by CT, work on dedicated reconstruction algorithms such as iterative reconstruction techniques that optimize the contrast-to-noise ratio (CNR) might help further reduce radiation exposure (Gramer et al. 2012).
2.1 Static or Dynamic Imaging for Cardiac CT Perfusion?
As compared to the rather challenging dynamic CT perfusion protocol, a single acquisition after contrast administration and adenosine stress may represent a promising alternative option to define myocardial contrast distribution at a predefined point in time at a relatively low level of radiation exposure. Given the high image quality of the latest high-pitch acquisitions, potentially resulting in radiation exposure of less than 1 mSv, it can be assumed that multiple scans, performed at different timing, may extend the small diagnostic window for delayed contrast distribution using such single-shot techniques. However, static imaging at one given time point—be it by single-source/single-energy or by dual-source/dual-energy techniques—will only allow for depiction of iodine distribution in the myocardium at a given time. Hence, the analysis of myocardial attenuation acquired at a single phase of arterial contrast enhancement without continuous time-resolved image acquisition enables detection of myocardial blood pool deficits but does not allow quantification of perfusion (George et al. 2006, 2007; Ko et al. 2012a, b; Blankstein et al. 2009).
As a perspective for these kinds of acquisition protocols, using the specific spectral characteristics of iodine and myocardium when exposed to different X-ray energy levels, Dual-energy acquisitions have been suggested as an alternative to quantify myocardial iodine concentration (Ruzsics et al. 2009a, b). It remains unclear at which point in time the acquisition should be triggered, as the inflow of contrast is clearly dependent on many factors. Using the data derived from dynamic, time-resolved CT perfusion datasets, Bischoff and colleagues have specifically defined the most valid acquisition time for the detection of ischemia, comparing opacification of ischemic versus nonischemic myocardium over time (Bischoff et al. 2012). Under pharmacological stress using adenosine, a maximum mean HU difference between ischemic and nonischemic myocardium (ranging from 17.7 to 22.5 HU) was observed 24–32 s after injection of contrast medium. Still, the optimal time point for a single-shot acquisition will certainly still be dependent on contrast injection, cardiac output, and scan parameters.
2.2 Do We Need a Quantitative Myocardial Perfusion Analysis?
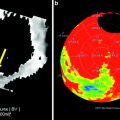
The main advantage of time-resolved dynamic CT imaging of the myocardial perfusion yields the promise of absolute perfusion quantification. As outlined above, first-pass acquisitions are limited either to the pure visual assessment of hypo-enhanced myocardial areas and/or density/iodine uptake measurements, and do not allow an exact quantification of myocardial perfusion. The typical quantitative parameters that can be calculated from a dynamic time-density curve as acquired by a dynamic perfusion protocol are the MBF and the myocardial blood volume (MBV) (see Fig. 2). The MBF scale units are ml/min/g, and the MBV scale units are ml/g. As 3D dynamic acquisition of the heart has recently become possible, the question must be raised, is absolute quantification of myocardial perfusion by CT needed at all, given the fact that these protocols are technically more challenging and less radiation dose effective as compared to single-phase protocols? Ultimately, the goal for cardiac perfusion imaging is to improve diagnostic accuracy for identifying flow-obstructing stenosis compared with CTA and CT perfusion alone and to provide information on the hemodynamic functional relevance of detected lesions.
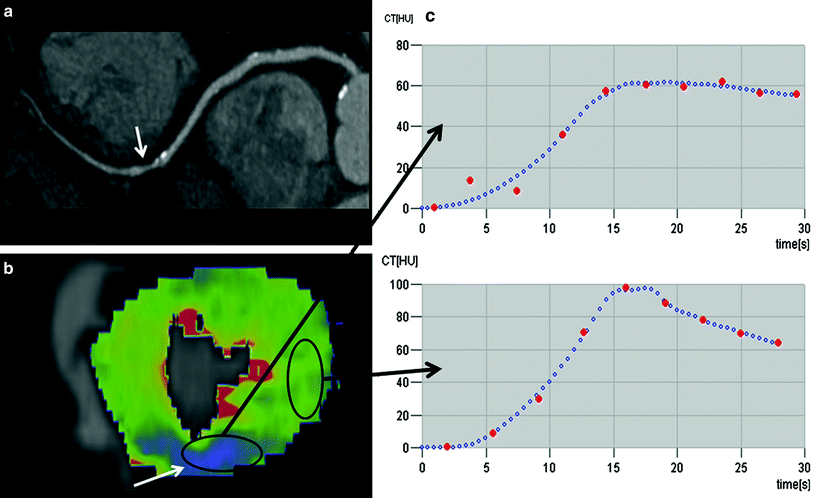

Fig. 2
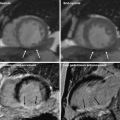
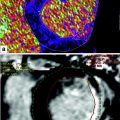
The multiplanar reformat of a patient with suspected coronary artery disease (CAD) of the right coronary artery (RCA) shows a potentially hemodynamically significant stenosis in the middle segment (a, arrow). A dynamic perfusion scan (color-coded parameter map) of the same patient depicts a perfusion defect under Adenosine stress in the RCA territory (b, arrow). From these data, time-density curves can be derived for under perfused (c, upper curve) and normal myocardium (c, lower curve), and quantitative perfusion values, such as the myocardia blood flow (MBF), can be calculated
In a clinical setting, the hemodynamic significance of a given coronary artery stenosis should be defined with invasive fractional flow reserve (FFR) measurements using a flow wire, representing the currently accepted standard for assessing the hemodynamic significance of coronary artery stenotic lesions (Tonino et al. 2009; Serruys et al. 1997). While CT provides a useful noninvasive technique to evaluate coronary artery anatomy, the functional significance of many coronary artery CT findings is often unclear and may lead to increased downstream test utilization (e.g., radionuclide perfusion imaging, stress MR imaging, or FFR) to help guide treatment decisions (Tonino et al. 2009; Shaw et al. 1999; Blankstein et al. 2010). Specifically, Meijboom et al. (2008) showed on the one hand that the diagnostic accuracy of a quantitative assessment of coronary artery stenosis with CT was highly correlated with angiographic findings. However, for the detection of hemodynamically significant coronary artery stenosis, as assessed with FFR, CT angiography only had a sensitivity of approximately 50 %. Thus, the combination of morphologic imaging with perfusion CT could ameliorate this diagnostic insufficiency of morphologic CT imaging alone (Bamberg et al. 2011).
Still, until dynamic myocardial CT perfusion imaging can be introduced to clinical routine, a number of questions need to be answered; such as, can a single perfusion scan under stress conditions differentiate between nonviable myocardium and reversible ischemia? Recent publications on dynamic cardiac CT perfusion imaging have acquired data at stress only and have not included additional dynamic perfusion imaging at rest, as protocols are designed to minimize the total radiation exposure. Acquiring two dynamic acquisitions, radiation dose would often exceed 20 mSv equivalent dose per patient. A solution to this missing rest perfusion dataset could be to use the dedicated coronary CT angiographic acquisition under resting conditions to assess first-pass myocardial perfusion. In this acquisition, a perfusion deficit would typically reflect nonviable myocardium or a high-grade stenosis inducing perfusion defects even at resting conditions. The second stress perfusion dynamic dataset would allow for identification of perfusion defects induced by significant stenosis not leading to myocardial iodine filling defects under resting conditions. Further, focused research will be necessary to determine which optimized combination of CT protocols will allow for differentiation between infarcted and ischemic myocardium by using either first-pass “static,” dynamic rest perfusion imaging, or delayed enhancement acquisitions (Vliegenthart et al. 2012).
Another question to be solved is which cut-off value of a quantitative parameter, such as MBF, should be used to optimize the differentiation of normal myocardium from myocardium with reduced perfusion. In a recent publication by Bamberg et al., an MBF of 75 mL/100 mL/min was determined as an ideal cut point, on the basis of maximization of the area under the curve (ROC analysis, detection of significant coronary artery disease as defined by FFR) as a criterion (Bamberg et al. 2011). However, this factor may need further validation across different patient populations. For instance, in subjects with an average MBF higher than this threshold level, findings could be incorrectly categorized as having a nonhemodynamically significant stenosis, even if the stenosis might be significant, i.e., leading to false-negative results. This fact could potentially lead to a decreased negative predictive value (NPV) for dynamic stress perfusion imaging. Thus, the optimal MBF threshold might be depending on the individual overall myocardial perfusion and should be adapted individually.
The inherent advantages of dynamic CT perfusion imaging over static imaging or established imaging modalities, such as scintigraphic techniques, are obvious. On the one hand, dynamic CT perfusion imaging might still be helpful in patients suffering from a multi-vessel CAD, as quantitative parameter maps comprising the whole myocardium in a 3D dataset should help to correctly identify disease by low MBF values in several vessel territories. Here, static single-shot techniques displaying only the relative iodine distribution in the myocardium might result in false-negative findings, as iodine contents in several areas of the myocardium could be homogeneously decreased in a multi-vessel CAD. Furthermore, smaller perfusion defects due to small vessel disease have been reported to be visible on dynamic CT perfusion datasets but cannot be seen scintigraphically or in imaging of the large vessels (with either catheter angiography or CTA) (Schuijf et al. 2006; De Bruyne et al. 2001). This high spatial resolution of CT perfusion is an inherent advantage, however, in a scientific setting comparing CT perfusion to other established imaging techniques, this fact may cause a real CT perfusion defect to appear as a false-positive result. E.g., although SPECT imaging is well established for the evaluation of myocardial perfusion, it may not detect subendocardial, nontransmural perfusion abnormalities because of the limited spatial resolution of approximately 10 mm, and some of the apparent “false-positive” CT perfusion studies may show perfusion abnormalities that may be below the resolution of nuclear MPI (Kido et al. 2008).
In conclusion, investigators who perform further research will need to determine the added value of “dynamic” imaging with MBF quantification to “static” imaging (i.e., a single set of images obtained during early myocardial perfusion) and to determine whether the added radiation exposure associated with dynamic imaging can be offset by improved detection of ischemia, e.g., in patients suffering from multi-vessel CAD.
3 Available Study Data on Dynamic Cardiac CT Perfusion Imaging
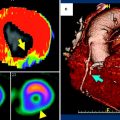
Several publications have reported on the diagnostic value and technical optimization of dynamic cardiac perfusion CT (see Table 2). In the first animal work involving five pigs with an induced 80 % LAD stenosis and using a dual-source CT with shuttle mode for dynamic perfusion imaging, Mahnken et al. showed the possibility of quantifying perfusion values and found significant differences between ischemic (related to an 80 % coronary area stenosis) and nonischemic myocardium (74.0 ± 21.9 versus 117.4 ± 18 mL/100 mL/min, p < 0.002) (Mahnken et al. 2010). If a scan situation in patients is to be simulated using an animal model, given their biological characteristics, the porcine model is known to be most similar to humans (Swindle et al. 1988). The weight of a pig’s heart is in a similar range, and injection of contrast material can be performed at a comparable rate to that used in humans, resulting in a similar opacification pattern of the left ventricle and myocardium. Accordingly, Bamberg et al. performed another in vivo animal study including seven pigs (Bamberg et al. 2012). Using different degrees of flow wire–adjusted induced coronary stenosis and different fluorescent colors, Bamberg et al. were able to simulate different perfusion conditions, both under rest and stress, demonstrating that a dynamic acquisition and derivation of MBF can depict differences in the tributary coronary artery territory similar to the distribution of microspheres in the corresponding histopathological correlation (i.e., 72 vs. 53 % for 75 % stenosis, rest versus stress) (see Fig. 3). The derived quantitative perfusion values were in line with early research on cardiac MRI (Patel et al. 2010).
Table 2




Available clinical study data on dynamic cardiac CT perfusion
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree