U. Joseph Schoepf, Fabian Bamberg, Balazs Ruzsics, Rozemarijn Vliegenthart and Gorka Bastarrika (eds.)Medical RadiologyCT Imaging of Myocardial Perfusion and Viability2014Beyond Structure and Function10.1007/174_2012_758
© Springer-Verlag Berlin Heidelberg 2012
CT Evaluation of the Myocardial Blood Supply: Technical Options
(1)
Department of Radiology, Duke University Medical Center, Durham, NC, USA
Abstract
The purpose of this book chapter is to provide an overview of novel technical advances and persistent challenges of coronary CT imaging targeting the evaluation of myocardial perfusion.
1 Introduction
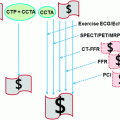
A large body of the literature has demonstrated the ability of coronary computed tomographic (CT) imaging to diagnose substantial coronary artery stenosis, suggesting the use of this test as a noninvasive alternative to conventional cardiac angiography in the work-up of patients suspected of having coronary artery disease (CAD) (Hoffmann et al. 2012; Litt et al. 2012). With use of the latest CT technology, it is now possible to image the entire heart during a single heartbeat, with substantial reduction of patient radiation exposure and motion artifacts. High-quality coronary CT studies yield a high level of diagnostic accuracy for the detection of CAD. In addition, recent evidence suggests that compared to a standard stress test evaluation, coronary CT may accelerate the diagnostic work-up of patients with acute chest pain in the emergency department.
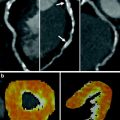
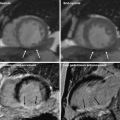
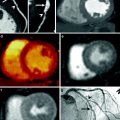
In addition to the anatomic assessment of the coronary artery lumen, another intriguing clinical application of coronary CT is the possibility of quantitative assessment of myocardial perfusion, allowing for a global anatomic and functional assessment of the heart in patients with CAD. Analysis of myocardial perfusion has important clinical implications for stratification of patients with acute CAD into different risk and management categories. For example, in patients with acute myocardial infarction the presence of areas of microvascular obstruction (i.e., areas of hypodensity relative to the surrounding myocardium during the early perfusion phase) and the extent of infarct size (defined by areas of delayed enhancement) correlate with an increased risk of complications, including the development of adverse left ventricular remodeling. When combined with the assessment of regional function, analysis of the early and late contrast-enhancement patterns also provides valuable information on tissue viability, and hence the likelihood of functional recovery after revascularization. Finally, myocardial perfusion assessment may provide the only valuable indicator for the identification of flow-limiting coronary artery stenoses in patients with limited quality coronary CTA due to motion artifacts and/or coronary calcifications.
In this chapter, we will briefly review important technical advances and persistent challenges for the application of coronary CT in the assessment of myocardial perfusion. The following chapters of this book will elucidate additional details of the individual strategies of different vendors for the assessment myocardial perfusion.
2 Improvements in Temporal Resolution
Coronary artery motion, which becomes particularly detrimental at increased heart rates (above 75 beats per minute), is a leading cause of suboptimal or clinically nondiagnostic coronary CT exams. To minimize the risk of cardiac motion, coronary CT should be performed using the best possible temporal resolution allowed by the CT scanner. Several strategies are available to reach this goal (Primak et al. 2006). One of the most effective strategies to decrease CT acquisition time is to minimize the number of projections used to reconstruct the image, including only those projections gathered in the shortest possible time window for image reconstruction. The minimum amount of data required to reconstruct a CT image is 180° plus the angle (in degrees) of the X-ray beam in the plane of the image (known as the fan angle). The effective data acquisition time—to acquire the necessary data to reconstruct an image of a single section—is therefore directly proportional to the rotation time of the gantry. However, faster gantry rotation requires a slower pitch in cardiac acquisition mode to avoid discontinuities in the anatomic coverage of the heart between images reconstructed from consecutive cardiac cycles. In addition, significant development efforts are needed to account for the substantial increase in mechanical forces (∼17 G for 0.42 s rotation time, ∼28 G for 0.33 s rotation time) and increased data transmission rates using faster gantry rotation times. For example, rotation times of less than 0.2 s (mechanical forces >75 G), which are required to provide a temporal resolution of less than 100 ms independent of the heart rate, appear to be beyond today’s mechanical limits.
One option to partially overcome the technical limitations for CT image reconstruction, while improving temporal resolution for coronary CTA, is the development of a scanner design with multiple X-ray sources and detectors. The dual-source CT systems (SOMATOM Definition and SOMATOM Definition Flash, Siemens Healthcare, Forchheim, Germany) are the first and second generation of these new concept designs. Equipped with two X-ray tubes and two corresponding detectors mounted onto the rotating gantry with an angular offset of approximately 90°, dual-source CT systems can combine the data from the two separate X-ray sources and detectors, virtually eliminating the need for multisegment reconstruction techniques for coronary CT (Petersilka et al. 2008; Flohr et al. 2006). With dual-source CT technology, the half-scan sinogram using a standard parallel geometry of a conventional single-source CT system can be split up into two quarter-scan sinograms, which are simultaneously acquired by the two acquisition systems in the same relative phase of the patient’s cardiac cycle and at the same anatomical level due to the ~90° angle between both detectors. The two quarter-scan segments are appended by means of a smooth transition function to avoid streaking or other artifacts from potential discontinuities at the respective start and end projections (transition angle 30°). With this approach, constant temporal resolution equivalent to a quarter of the gantry rotation time is achieved in a centered region of the scan field of view. Data from one cardiac cycle only are used to reconstruct an image, thus minimizing the risk of motion artifacts between consecutive heartbeats. This approach is radically different from that of other conventional single-source CT systems, which can theoretically provide similar temporal resolution by means of multisegment reconstruction approaches. With these approaches, temporal resolution strongly depends on the heart rate; a stable and predictable heart rate and complete periodicity of the heart motion are required for adequate performance.
Dual-source CT systems are also compatible with multisegment reconstruction techniques, which may result in interesting applications in particular clinical settings. Using a two-segment reconstruction, the quarter-scan segments acquired by each of the two detectors are independently divided into smaller sub-segments acquired in subsequent cardiac cycles—similar to two-segment reconstruction in conventional single source CT. The temporal resolution of multisegment reconstruction techniques is directly affected by the patient’s heart rate, with an average temporal resolution of 60 ms using a 0.33-s gantry rotation time (minimum temporal resolution 42 ms). While multisegment reconstruction techniques using a dual-source CT are generally not recommended for coronary CTA examinations, this approach may be beneficial for advanced functional analysis, such as the assessment of myocardial wall perfusion, wall motion abnormalities, or the determination of cardiac function indices, such as cardiac chambers volumetric analysis and ejection fraction evaluation.
The recent introduction of second generation of dual-source CT systems with the use of two 128-section detector rows have further improved temporal resolution for coronary CTA, introducing new strategies for faster acquisition times. Using previously unexplored high pitch settings (beam pitch up to 3.4, corresponding to a table speed of 458 mm/s), this new system allows ultrafast CT acquisition, allowing prospective ECG-triggered scanning of the heart within a single heartbeat with continuous movement of the table during scanning (Flohr et al. 2009; Achenbach et al. 2009). The high pitch acquisition technique also yields substantial reduction of patient radiation dose, due to virtual elimination of partial overlap during spiral CT acquisition (Achenbach et al. 2010). While very promising, the high pitch technique may not be adequate for larger patient populations or highly attenuating anatomical areas, such as the abdomen and pelvis. This is based on the technical limitations in the maximum tube current output of the currently available X-ray tubes using high pitch settings, which in turn may result in increased image noise and clinically unacceptable image quality.
Recently, a novel vendor-specific motion-correction algorithm (SnapShot Freeze; GE Healthcare, Waukesha, Wis) has been developed that uses information from adjacent cardiac phases within a single cardiac cycle to characterize and correct for cardiac motion. By using information obtained from adjacent pixels, this approach may correct for the complex and elastic motion deformations in the heart and coronaries during the cardiac cycle. This effect may result in potentially motion-free, high quality coronary CT images. Preliminary phantom studies suggest that blurring secondary to cardiac motion can be decreased by a factor of six with this new motion correction algorithm, which would correspond to an effective gantry rotation speed of 0.058 s (58 ms) (Scott Schubert 2012). Additionally, early clinical experience demonstrates that this motion correction algorithm significantly improves image quality, interpretability, and diagnostic accuracy of coronary CTA studies without the use of rate control medications (Leipsic et al. 2012).
3 Dual-Energy CT Applications
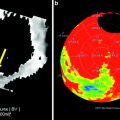
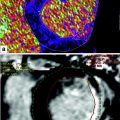
For decades, dual-energy imaging has been used for tissue differentiation with several X-ray-based imaging modalities, exploiting the fact that the tissues in the human body show different absorption characteristics when penetrated with different X-ray energy spectra at different kV settings of the X-ray tube (Coursey et al. 2010). The ability to separate different materials using dual-energy may substantially improve the interpretation of myocardial perfusion CT studies by providing enhanced information on iodine distribution within the myocardium with the additional help of color-coded iodine distribution maps. These techniques may result in improved diagnostic accuracy for the detection of areas of decreased or absent perfusion caused by flow-limiting coronary stenosis (Ruzsics et al. 2008, 2009).
Although early work in the 1970 and 1980s demonstrated that dual-energy CT technology improved tissue characterization, its utility was limited because of noise in the low-kilovoltage images and the amount of time required for data acquisition, which led to misregistration. Newer CT technologies allow for more rapid data acquisition and have sparked renewed interest in dual-energy applications. Manufacturers continue to improve dual-energy CT scanners, and those that are currently available differ in terms of the number of X-ray tubes, the number and arrangement of detector arrays, the energy of fan beams, and the rotation of X-ray tubes and detector arrays. Current dual-energy CT scanners also offer improved temporal resolution, which is helpful for coronary CT applications, and increased photon flux, which is critical for imaging diagnostically challenging larger patient populations.
The dual-source CT scanners (SOMATOM Definition and SOMATOM Definition Flash, Siemens Healthcare, Forchheim, Germany) allow operation of both X-ray tubes at different kV and mA settings, with the possibility of acquiring closely co-registered dual-energy data. Another major advantage of using a dual-source approach for dual-energy acquisition is the possibility of using different filtration for independent shaping of the two X-ray spectra (Primak et al. 2009). The X-ray spectra generated at low (80 kV) and high (140 kV) peak tube potentials have a high degree of spectral overlap, resulting in a separation between the average energies of the two spectra much less than the 60 keV difference in peak potential. The ability of DECT to discriminate between two materials relies primarily on the difference between the energy ratio of the materials, which is determined by the separation between the high- and low-energy spectra and the difference between the effective atomic numbers of the evaluated materials. The smaller the spectral separation, the harder it is to discriminate between two materials, especially for materials with close atomic numbers (e.g., calcium and iron).
Using additional filtration for one or both tube potentials (kV) can increase spectral separation, resulting in increased dual-energy contrast between different materials (Leschka et al. 2010; Primak et al. 2010). This effect can significantly enhance the performance of dual-energy material decomposition algorithms designed to discriminate between different materials, such as calcium (e.g., bone or calcified plaque) and iodinated contrast material. This effect may result in improved sensitivity of a dual-source dual-energy CT technique for detecting areas of different myocardial perfusion due to ischemia. In addition, improvements in material decomposition using dual-energy may also result in better discrimination between calcium and iron, which are indistinguishable using conventional magnetic resonance imaging (MRI) and single-energy CT techniques. Detection of iron deposition within a coronary plaque has important clinical implications as intraplaque hemorrhage has been advocated as an early indicator of plaque vulnerability, possibly allowing lesion early intervention before acute plaque complications and myocardial infarction.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree