Stroke is the third-leading cause of death in the United States. It occurs in almost 700,000 people per year and cost an estimated $57.9 billion in 2006. Atherosclerotic disease is the cause of one third of these strokes, with more than one half of these stenoses being extracranial in location. Carotid stenoses are usually unifocal and 90% occur within 2 cm of the carotid bulb. Currently, carotid endarterectomy accounts for 117,000 surgical revascularizations per year, whereas carotid angioplasty and stenting are performed less than 10,000 times annually. Stenoses amenable to revascularization are the topic of this article.
Stroke is the third-leading cause of death in the United States . It occurs in almost 700,000 people per year and cost an estimated $57.9 billion in 2006 . Atherosclerotic disease is the cause of one third of these strokes, with more than one half of these stenoses being extracranial in location . Carotid stenoses are usually unifocal and 90% occur within 2 cm of the carotid bulb . Stenoses amenable to revascularization are the topic of this article.
Currently, carotid endarterectomy (CEA) accounts for 117,000 surgical revascularizations per year, whereas carotid angioplasty and stenting (CAS) are performed less than 10,000 times annually . Endovascular therapy for extracranial carotid stenoses has become an increasingly attractive alternative to surgery. Much of this interest is fueled by advances in technology and technique. Similarly, knowledge of the natural history of lesions treated by endovascular means, and the immediate periprocedural complications of intervention, have contributed greatly to the interest in minimally invasive techniques.
Advances in endovascular interventional techniques and devices have been rapid. The development and improvement of distal embolic protection devices (EPDs) and rapid exchange systems allow the endovascular operator rapid and safe access to the carotid lesion. The temporary proximal carotid occlusion required for endarterectomy is now avoidable when using endovascular techniques. Because extracranial carotid disease often occurs in the face of systemic disease, the surgical risk to these patients can be high . The minimal invasiveness of CAS makes it an option for these high-risk patients, much in the way coronary angioplasty has become a viable alternative to bypass surgery. Shorter hospital stays, shorter procedural and carotid occlusion times, and freedom from general anesthesia all contribute to the theoretic decrease in procedural risk.
Indications
One of the major difficulties in CAS has been establishing a consensus on the clinical indications for treatment. Endarterectomy became the basis of treatment after the North American Symptomatic Carotid Endarterectomy Trial (NASCET) , the European Carotid Surgery Trial (ECST) , and the Asymptomatic Carotid Atherosclerotic Study (ACAS) in the 1990s. In the past, many endovascular trials adopted ECST and ACAS exclusion criteria as the basis for inclusion in endovascular trials. As a result, most practitioners had adopted only those criteria as clinical indicators for treatment, until the last several years.
Patients with normal risk
In the normal surgical risk population, the NASCET investigators demonstrated that endarterectomy for high-grade symptomatic carotid stenosis (70%–99%) was better than medical management . The absolute risk of major or fatal ipsilateral stroke at 2 years was reduced by 10% by endarterectomy. Since the publication of this data in 1991, the clinical indications for endarterectomy have evolved. The ECST demonstrated that CEA provided benefit in patients with symptomatic lesions greater than 50%. As a result, endarterectomy is performed on this group of normal-risk patients in most clinical vascular surgery practices. In addition, many patients who would have been considered at higher surgical risk (see later discussion) according to ACAS and NASCET criteria are now undergoing endarterectomy, according to Medicare analysis .
The indications for treating asymptomatic carotid lesions are not as clear-cut. The authors of ACAS demonstrated that asymptomatic lesions greater than 60% benefited from endarterectomy as long as the overall surgical risk was less than 3%. The study design has been criticized for the exclusion of patients with comorbidities, who would have otherwise done poorly. Thus, the population selected was not representative of the patient routinely treated with endarterectomy in clinical practice or his/her operative mortality . Another major criticism is that the data may be outdated with regards to advances in medical management, such as more effective antiplatelets and statins .
For normal-risk patients, no data suggest CAS is equivalent to CEA in either symptomatic or asymptomatic patients. This field is a topic of ongoing study and debate. Two highly publicized studies attempted to prove that CAS is not inferior to CEA, but failed to do so. Both studies used a noninferiority design, which has the advantage of requiring less numbers than a standard comparison. In the EVA-3S trial, the investigators compared CEA to CAS in symptomatic severe carotid stenosis in normal-risk patients . The study was stopped early because of increased 30-day risks of stroke or death in the CAS group and the inability to enroll the numbers needed to power a study proving equivalence. In the SPACE trial, the investigators failed to prove noninferiority in normal-risk patients who had moderate-to-severe stenosis. Although the study was not stopped early, they also concluded that they could not enroll the numbers needed to prove equivalence . Although both studies failed to show noninferiority, readers should be cautioned not to interpret the data to suggest that CEA is, in fact, superior to CAS. Ultimately, a large, randomized trial, such as the National Institutes of Health–funded Carotid Revascularization Endarterectomy versus Stent Trial (CREST), is needed to power a true comparison .
High-risk patients
The issue of the best treatment modality for high-risk patients who have symptomatic stenosis is also a topic of ongoing study and debate. As mentioned earlier, the ECST and ACAS exclusion criteria formed the basis of inclusion in CAS trials, mainly patients with high surgical risk. In total, only three prospective studies analyzed CAS outcomes using each of the three stents currently approved by the Food and Drug Administration . Of these, only one was a randomized clinical trial, the Stenting and Angioplasty with Protection in Patients at High Risk for Endarterectomy (SAPPHIRE). This study is the only randomized clinical trial comparing CAS with CEA in high-risk patients. Its inclusion criteria, asymptomatic lesions greater than 80% and symptomatic lesions greater than 50% in high-risk patients, resulted in the current reimbursement authorization by the Centers for Medicare and Medicaid Services (CMS), and are the basis for ongoing trial registries ( Box 3 ). This authorization, in turn, has made its impact on patient selection for treatment in clinical practice. The 1998 American Heart Association (AHA) expert consensus panel defined the medical and anatomic conditions considered risk factors associated with increased complications after CEA ( Box 2 ), which have been adopted since, for purposes of CMS reimbursement.
If high surgical risk and symptomatic with stenosis of
>70%: authorized a
50%–69%: must be enrolled in category B IDE clinical trials or postapproval study b
If high surgical risk and asymptomatic with stenosis of
≥80%: must be enrolled in category B IDE clinical trials or postapproval study b
a Coverage limited to Food and Drug Administration–approved devices.
b as a routine cost per Medicare National Coverage Determinations Manuals 20.7 and 310.1, Regulation 42 CFR 405.201sz.
Anatomic issues
Contralateral carotid occlusion
Contralateral laryngeal nerve palsy
Lesion below clavicle
Prior ipsilateral carotid endarterectomy
Prior radical neck dissection or irradiation
Stenosis at odontoid (C2) or higher
Tracheostomy
Neck anatomy impeding surgical exposure (unable to extend, short, or obese)
Medical issues
Age over 80 years
Class III/IV congestive heart failure
Class III/IV angina pectoris
Left main, or two or more vessel, coronary artery disease
Recent/urgent heart surgery (<30 days)
Severe chronic pulmonary disease
Severe renal disease
CLASS I, Level A evidence
Recent transient ischemic attack or ischemic stroke within the last 6 months AND ipsilateral severe (70%–99%) stenosis, CEA is advised IF surgeon has a perioperative morbidity and mortality rate of <6%
Recent transient ischemic attack or ischemic stroke within the last 6 months AND ipsilateral moderate (50%–69%) stenosis, CEA is advised IF favorable patient factors such as younger age, male gender, less severe presenting symptoms (hemispheric versus retinal, and so forth)
CLASS IIa, Level B evidence
CEA is indicated, recommend surgery within 2 weeks rather than delayed procedure
CAS is reasonable to consider if performed by an operator with a periprocedural morbidity and mortality rate of 4% to 6%, similar to that observed in CEA and CAS trials
CLASS IIb, Level B evidence
CAS is not inferior to CEA and should be considered IF symptomatic severe stenosis (>70%) and stenosis presents a difficult surgical access OR medical comorbidities are present that increase the surgical risk OR contraindications, such as radiation-induced stenosis after CEA
CLASS III, Level A evidence
When stenosis is <50%, CEA is not indicated
As mentioned earlier, a large, prospective, randomized trial comparing CEA with CAS is needed. The National Institutes of Health–funded CREST is ongoing and randomizing 1400 symptomatic, normal-risk patients who have greater than 50% stenosis by angiography or greater than 70% by ultrasound, and 1100 asymptomatic, normal-risk patients who have greater than 60% stenosis by angiography or greater than 70% by ultrasound. The International Carotid Stenting Study (ICSS), also known as CAVATAS-2, is also an ongoing, multicenter, randomized trial with a planned enrollment of 1500 patients . The study plans to compare the two techniques in normal-risk patients who have symptomatic severe stenosis.
Current recommendations
Only recently has expert consensus attempted to clarify the indications for CAS. The document is the American Heart Association/American Stroke Association (AHA/ASA) Recommendations for Revascularization in Symptomatic Patients, ( Box 3 ) , which recommends CAS only for symptomatic lesions greater than 70% in high-risk patients. As a result, CMS now authorizes reimbursement for high-risk symptomatic patients who have greater than 70% stenosis. It also authorizes reimbursement for high-risk patients who have symptomatic lesions greater than 50% and asymptomatic lesions greater than 80% when enrolled in a Category B investigational device exemption (IDE) clinical trial or postapproval study (see Box 1 ). Ultimately, this reimbursement authorization has become the driving force of patient selection in clinical practice.
Other considerations
Many nuances to an individual’s condition may preclude endovascular revascularization, despite meeting the inclusion criteria discussed earlier. In some of these situations, the exclusionary aspect may not become apparent until the patient has presented to the angiography suite and been catheterized. The absolute and relative contraindications can be grouped into neurologic, medical, and anatomic considerations ( Box 4 ).
Clinical
Contraindication to aspirin or thienopyridines
Life expectancy <5 years
Renal dysfunction precluding IV contrast administration
Anatomic
Heavily calcified lesion, especially if concentric
Intracranial aneurysm or arteriovenous malformation requiring treatment
Severe common or internal carotid artery tortuosity
Total or long subtotal occlusion “string sign”
Visible thrombus within lesion
No safe endovascular access
Neurologic
Major functional deficit
Major stroke within last 4 weeks
Significant cognitive impairment
Neurologic considerations
The neurologic considerations relate mostly to long-term quality of life. Because many potential candidates may be older and have long-standing hypertension, some degree of intracranial disease is inevitable. Whether the burden of ischemic white-matter disease is significant enough to impair cognition is unique to that individual. No randomized controlled trials have evaluated the long-term improvement in quality of life in patients who have cognitive impairment. As the degree of cognitive impairment increases, so does the confidence that CAS will not improve functionality or long-term morbidity. Thus, it is reasonable to consider “severe” cognitive functional impairment a contraindication to CAS.
Currently, major stroke within 4 weeks is a relative contraindication. However, acute strokes are sometimes the exception to this rule. These strokes can involve collateral failure or artery-to-artery embolization, and these special circumstances are discussed in detail in the last section.
Anatomic considerations
Anatomic considerations relate to the degree of underlying age-related and chronic hypertensive changes in the vascular system and the structural characteristics of the stenosis. Safe vascular access is required before the start of any angiographic procedure. Chronic hypertensives and vasculopaths are at risk of developing peripheral vascular disease, iliac artery stenoses, and aortic aneurysms. Alternatively, they may have a history of surgical correction for these conditions, both of which may make catheterization difficult or hazardous.
After vascular access is obtained, being able to navigate to the lesion in question can be difficult. Age and chronic hypertension can alter thoracic and cervical vascular anatomy, which makes catheterization difficult. An unwound aorta and tortuosity of the origins of the innominate and left common carotid arteries may be severe enough to preclude CAS. In certain situations, where takeoff angles are severe, brachial or radial access may be an alternative, particularly in regards to the origin of the right common carotid artery or a bovine origin of the left common carotid. The cervical portions of the common and internal carotid arteries may be tortuous themselves, allowing for navigation of a microguidewire but not the stiffer stent delivery systems. Factors such as the tortuosity of the brachiocephalic system or a type III aortic arch (when the origin of the innominate artery is caudal to the inner inferior curve of the aortic arch) may make it difficult to maintain access with the guiding catheter. Such difficulty may also lead to prolonged EPD deployment time, which has been shown to be an independent risk factor for procedural complications .
The morphology of the lesion itself should be analyzed before pursuing CAS. A heavily calcified or circumferential lesion may be a relative contraindication. Because calcification is very resistant to balloon dilatation, the radial force from a self-expanding nitinol stent is also likely to be insufficient. Visible thrombus as a component of an atherosclerotic lesion is also a contraindication. The exception, as discussed in the last section, is acute stroke treatment.
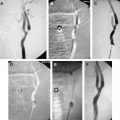
Patients have a lower risk of ipsilateral stroke in the territory of a carotid artery if it is nearly or totally occluded . Thus, the presence of a “string sign” by MR angiography or carotid Doppler ultrasonography (DUS) is a relative contraindication. In severe stenoses that may not qualify as near-occlusion, one of the considerations is vessel collapse. If little forward perfusion pressure is seen across a large gradient created by the stenosis, the distal carotid can be collapsed significantly ( Fig. 1 ), which may pose a problem with distal EPD selection. The smallest EPD currently available is 2.0 mm in diameter. If the anteroposterior (AP) dimension is collapsed enough not to accommodate such an EPD, CAS may not be possible and may be hazardous if performed without one. Additionally, should a distal EPD fit properly, it may not be sufficient to protect from distal embolization of debris when the gradient is normalized, forward perfusion pressure is restored, and the normal diameter of the distal vessel is restored, producing a situation where the initial EPD selection is undersized.
Medical considerations
Certain medical conditions exist that may be contraindications to CAS. Renal insufficiency should be evaluated carefully. Postponement of intervention may be required to allow for medical and intravascular volume optimization and to reduce the risk of contrast nephropathy . If CAS is absolutely necessary, the patient and family should be informed of the possibility of impending hemodialysis, which can potentially be lifelong, altering the risk/benefit ratio considerably.
Life expectancy is an important consideration when calculating the risk/benefit ratio for CAS. In asymptomatic patients with a less than 5-year life expectancy or symptomatic patients with a less than 3-year life expectancy, CAS may be contraindicated, based on the ACAS and NASCET data .
Post-CAS management has adopted cardiology recommendations for antiplatelet therapy to prevent stent luminal thrombosis. Any contraindication to the use of antiplatelets similarly precludes CAS as an option. However, the patient’s ability to tolerate a single agent may be sufficient to proceed. No evidence at this time suggests that the use of double antiplatelet therapy is superior to a single agent in preventing cerebrovascular ischemic events , whereas the use of double antiplatelet therapy in preventing endovascular stent restenosis is common in clinical practice , but lacking Level I evidence.
Age is a controversial contraindication in carotid revascularization. In observational studies, age older than 80 was an independent risk factor for post-CEA stroke . This age cut-off has also been adapted by the American College of Cardiology as a contraindication to CEA . Although SAPPHIRE did not exclude octogenarians and, in fact, showed fewer complications with CAS than CEA, CREST suspended enrollment of this group because of a higher risk in its lead-in phase of the study . However, the randomized phase of CREST has no age limit, in keeping with the NASCET design. At present, no Level I evidence indicates age older than 80 is a risk factor for post-CAS complications.
Gender is also a controversial topic in the CEA literature. NASCET showed less long-term benefit of surgery for women than men , whereas another study showed increased perioperative mortality in women . Subgroup analysis of NASCET data revealed that women had a decreasing benefit with increasing treatment delay from time of initial stroke . To date, however, no conclusive evidence exists that gender affects CAS outcome.
Other considerations
Many nuances to an individual’s condition may preclude endovascular revascularization, despite meeting the inclusion criteria discussed earlier. In some of these situations, the exclusionary aspect may not become apparent until the patient has presented to the angiography suite and been catheterized. The absolute and relative contraindications can be grouped into neurologic, medical, and anatomic considerations ( Box 4 ).