EYE: DEVELOPMENTAL ABNORMALITIES AND ACQUIRED ALTERATION IN THE SIZE AND SHAPE OF THE EYE
KEY POINTS
- An eye that is deformed or altered in size or somehow otherwise malformed may be associated with acquired or developmental disease, and a search for associated findings and conditions may be warranted.
- Imaging is most effective when the clinical circumstances are ambiguous, and imaging is likely to make the situation more clear especially by excluding a life-threatening disease entity.
- Such findings and diseases are often detected as incidental issues not really affecting the plan of care for the patients.
- Ultrasound, in the correct hands, can supplant much of the need for computed tomography or magnetic resonance imaging, and the latter should be used judiciously and only after other diagnostic testing by the ophthalmologist has been properly executed and factored into the medical decision-making process.
Developmental abnormalities of the eye and alterations in the size of the eye may be isolated findings. Eye anomalies are often associated with other findings involving the visual system and may also be seen as part of syndromes and other more generalized genetic errors. Once a potentially developmental abnormality of the eye is recognized, it becomes important to identify such possible related conditions and their likely origin.
Sometimes, the eye abnormality is an acquired disorder seen as result of a developmental problem. This range of eye size variation and focal abnormalities of shape will be covered in this chapter. This serves as a foundation for the discussion of congenital conditions presented here and both congenital and acquired conditions in other chapters.
ANATOMIC AND DEVELOPMENTAL CONSIDERATIONS
Embryology
The eye is derived from primordial neuroectoderm that forms the retina; neural crest mesenchyme contributing the uvea, sclera, and posterior vitreous; and cutaneous ectoderm giving rise to the lens placode. The optic vesicles form at 3 weeks of gestation as an eventration of the primitive forebrain1,2 (Fig. 46.1).
The only remnant of the optic vesicle origin in the mature brain is the optic recess of the third ventricle. Subsequently, each optic vesicle first invaginates distally to form an optic cup and optic stalk and then forms a cleft, the choroidal fissure, along its inferior nasal surface. The folding in or invagination of the distal optic vesicle creates the two layers of the retina referred to as the neural and pigmented layers. The proximal end of the optic vesicle creates the optic stalk that ultimately becomes the optic nerve.
The choroidal fissure allows the embryonic hyaloid artery and neural crest mesenchyme to access the internal aspect of the developing globe. The hyaloid artery perfuses the developing lens. Mesenchymal cells intermixed with the neural crest cells form the cellular matrix for the primary vitreous that fills the posterior chamber. Later, the primary vitreous is replaced by the mainly gel-containing secondary vitreous. The hyaloid artery regresses in the early part of the third trimester, leaving its embryonic remnant called the canal of Cloquet.1,3 Neural crest mesenchyme also forms the optic sheath, a duralike covering for the optic nerve. Neural crest cells are also the source of Schwann cells for intraorbital sensory and motor nerves, but no Schwann cells cover the optic nerve.
Mesoderm differentiates to create the ocular blood supply, including the long and short ciliary arteries and the central retinal artery. These arteries arise as branches of the ophthalmic artery, itself a branch of the internal carotid artery. The ophthalmic artery initially is an external carotid branch and is linked with development of the middle meningeal artery; variations exist, however, wherein the ophthalmic artery can arise from the middle meningeal artery and vice versa. This is a critical factor when planning angiography and endovascular therapy for head and neck lesions.
Each optic vesicle ultimately forms the inner layers of the retina, including the photosensitive neural layer and the deeper pigmented layers. Within the eye, the pigmented layer extends anteriorly to form the under surface of the iris, while the neural retina terminates at the ora serrata located at the base of the iris. The optic cup engulfs the developing lens, and the mouth of the optic cup becomes the pupil. Mesenchyme surrounding the developing optic cup differentiates to form the uveal tract, including the choroid, ciliary body, and iris and the sclera, including the cornea.
The visually active cells send their axonal processes retrograde within the optic stalk, filling the central cavity and forming the optic nerve. Axonal processes cross appropriately at the optic chiasm, synapse at the lateral geniculate body, and separate from the hypothalamus. Myelination begins prenatally and extends well into the postnatal period.
The remainder of normal ocular development requires that the choroidal fissure closes, that all primitive mesenchyme connected with the cornea and lens retract appropriately, and that the primary vitreous and the primitive hyaloid artery are replaced by the secondary vitreous and the central retinal artery. Normal ocular development is necessary for normal orbital development.
Applied Anatomy
The anatomy of the eye and orbit are discussed in detail in Chapter 44. The following section provides a working understanding of the scope of anatomic knowledge required for evaluating abnormal size and shape as well as developmental abnormalities of the eye.
The eyes are nearly spherical and usually symmetric in size and configuration. The usual degree of anterior to posterior (AP) length variation necessary to produce clinical myopia (longer globes) and hyperopia (shorter globes) are subtle and are usually not appreciated on imaging. Congenital variations, however, may be more grossly apparent. Asymmetry usually implies an underlying abnormality such as macrophthalmia or buphthalmos, staphyloma or coloboma; each of these entities is discussed subsequently. The scleral margins should not vary in thickness.
The uvea is composed of the highly vascular choroid, the ciliary body, and the iris. The entire uveal tract is seen on good-quality, contrast-enhanced magnetic resonance (MR) and computed tomography (CT) images. Excessive enhancement of the iris may not be normal, but this can be sorted out clinically. The enhancing choroid is smooth and thin, interrupted only at the optic nerve head. The optic disc should be flat.
The vitreous body is normally of fluid-equivalent signal intensity on T1- and T2-weighted images and fluid density on CT. It is normally homogenous and does not enhance. However, in cases of retinal inflammation and vitreous hemorrhage, the vitreous may enhance on a magnetic resonance imaging (MRI) scan. The eye is suspended in a soft tissue socket formed by reflections of fascia known as the Tenon capsule. The potential Tenon space lies between the eye and this cup.
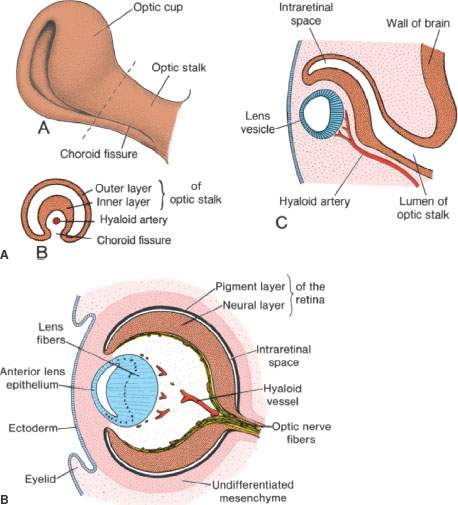
FIGURE 46.1. A–C: Basic developmental elements of the eye and optic nerve. In (B), the evolution of the primary to the secondary vitreous is shown—one important component of which is the hyaloid artery. This illustration shows the anatomic substrate for persistent hyperplastic primary vitreous. (From Sadler TW. Langhams Medical Embryology. 11th ed. Philadelphia, Pa: Lippincott Williams & Wilkins; 2009.)
IMAGING APPROACH
Techniques and Relevant Aspects
Ultrasound is generally performed with an 8- to 10-MHz transducer. Either A-mode or B-mode, color flow, and power Doppler techniques are used depending on specific indications.
CT and MRI protocols for studying the eye and orbit for various indications are explained in Chapter 44 and presented in Appendixes A and B.
Pros and Cons
For the eye, ultrasound with color flow Doppler techniques may be the only diagnostic imaging examination needed, although MR and CT can be used adjunctively or as a first choice depending on the clinical situation. When ultrasound is not definitive, MR is preferred if it is properly matched to the diagnostic task. CT should be avoided in children in favor of other techniques without ionizing radiation whenever possible. The main limitations of MR in evaluating the eye are its sensitivity to motion artifact and insensitivity to calcification.
Controversies
Few real controversies exist with regard to imaging of this anatomic region. If ultrasound is not brought into the decision-making process, the choice between CT and MRI as a starting point for a given diagnostic problem is usually fairly well defined.
Ultrasound is in reality a tool essentially of the ophthalmologist; few diagnostic radiologists perform or interpret these examinations. It is possible that ultrasound studies may be cost additive without definitive results in some cases, especially if one already knows that CT or MRI will be performed later in the diagnostic process. On the other hand, ultrasound examinations, in experienced hands and the correct clinical context, have the capacity to eliminate the need for CT and/or MRI with obvious resulting benefits.
SPECIFIC DISEASE/CONDITION
Microphthalmia and Anophthalmia
Microphthalmia is usually defined as a small eye affected by one or more structural abnormalities and may be differentiated from nanophthalmos, which is a subtype of microphthalmia where the globe is small (<20 mm in an adult) but has no gross structural abnormality. Microphthalmia and anophthalmia can be sporadic or inherited.4 Eyes may also be abnormally small or large because of acquired diseases that are considered in both this and other chapters. These conditions are a spectrum of abnormally small eyes ranging from anophthalmia, the very rare complete absence of the eye, to very minor degrees of decreased axial length of the eye.
Phthisis is the condition where the globe becomes small and shrunken often in association with thickened sclera, usually as an end-stage result of severe ocular disease such as endophthalmitis and trauma.
Smaller than normal eyes have a globe diameter <20 mm in adults and 19 mm in a 1 year old. Under 1 year of age, the globe may be 17 to 18 mm normally.
Prevalence and Epidemiology
These are relatively uncommon conditions, with true anophthalmia being rare since a tiny, dysmorphic remnant developmental cyst is normally found in cases of clinical anophthalmia. Abnormally small eyes may be seen in association with many syndromes and likely as a result of in utero toxic or infectious exposure leading to trophic or genetic mechanism of dysgenesis.
The most likely associated abnormalities are those of the limbs, face and ear, central nervous system, genitalia, oral clefts and then the rest of the body systems.
Clinical Presentation
Unilateral microphthalmic patients and those with anophthalmia will present with a small ipsilateral orbit and related facial asymmetry, variable visual deficits, or associated syndrome features, some suggested in the previous section. Patients with nanophthalmos usually have bilaterally small eyes with fairly normal function, although they are prone to certain complications as a result of the small globe size, such as angle-closure glaucoma and retinal detachment.
Pathophysiology and Patterns of Disease
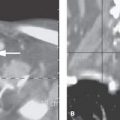
Microphthalmic eyes in adults have an axial diameter <20 mm, with that number dropping to <19 mm in a 1 year old. Unilateral or bilateral microphthalmia may be due to developmental issues. Unilateral microphthalmia may be due to acquired disease such as Coats disease or developmental conditions such as persistent hyperplastic primary vitreous (PHPV) (Fig. 46.2). Microphthalmia from a developmental perspective can be the result of abnormalities of optic cup formation and/or closure of the choroidal fissure; therefore, microphthalmia is often seen in conjunction with coloboma. Ocular hypoplasia is present in many cases with coloboma, and many of these patients have associated intracranial or maxillofacial anomalies5 (Fig. 46.3). Trisomy 13, thalidomide, and LSD embryopathy frequently exhibit coloboma and microcephaly.

FIGURE 46.2. Two patients with persistent hyperplastic primary vitreous (PHPV) and secondary microphthalmia. A: Patient 1 with unilateral PHPV. The persistent primary vitreous is seen as a retrolental soft tissue mass and a central canal between the lens and the optic nerve (hyaloid remnant, Cloquet canal) in a small eye. Hyperintense signal of the vitreous chamber is due to hemorrhage in the subretinal or subhyaloid spaces. B, C: Patient 2 with severe bilateral PHPV. Bilateral microphthalmos with small anterior chambers and a retrolental soft tissue mass extending centrally to the optic nerve is seen. The vitreous chamber shows mixed signal intensities on both T1-weighted (B) and T2-weighted (C) images due to different episodes of hemorrhage with a fluid level on the left reflecting serosanguineous fluid in the subhyaloid or subretinal space (arrow).

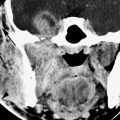
FIGURE 46.3. A: Non–contrast-enhanced computed tomography study showing bilateral perioptic colobomas (arrows). Note the microphthalmic eye on the right. B: A patient with a small orbit and microphthalmia on the left side. The eye itself is small perhaps with a somewhat thickened choroid (arrows) and clear evidence of abnormal enhancement of the ciliary bodies and iris (arrowhead). This microphthalmic eye is associated with brain atrophy in the left temporal lobe. C–E: A patient with colobomas (arrowheads) and orbital cysts (arrows) in (C) and (D) and associated holoprosencephaly (E) and small midface (F).
Reduced eye size or even phthisis bulbi can also be the result of endophthalmitis, trauma, or exudative retinopathies (Figs. 45.4 and 46.4). These usually have dystrophic calcification and fibroproliferative change within the eye as well. Microphthalmia may be associated with other orbital cystic lesions such as epidermoid/dermoid developmental cyst and meningoencephalocele5 (Fig. 46.5).
Anophthalmia is complete absence of the eye as confirmed by histologic examination of orbital contents. Anophthalmia is the result of failure to develop an optic vesicle. Failure to make two distinct and separate optic vesicles creates the anomaly of cyclops. This is a rare condition that is readily apparent, both clinically and on imaging. Orbital hypoplasia will result from anophthalmia (Fig. 46.6).
Manifestations and Findings
Plain Film and Fluoroscopy
The bony orbit usually appears abnormally small in advanced cases. The orbit could be paradoxically large if there is an associated mass or cyst within the orbit. Manifestations of associated facial dysplasia, clefts, and other malformations that are either syndromic or nonsyndromic may be present.
Computed Tomography
The same manifestations of the bony orbit seen on plain films may be present with the same range of associations.
CT may show thickened sclera. It also may reveal associated coloboma, orbital cysts, and temporal bone and brain malformations depending on how much of the adjacent anatomic region is included.
CT would be the preferred method for screening for associated facial skeletal and temporal bone malformations once the presence of microphthalmia is established (Fig. 46.3F).

FIGURE 46.4. Acquired microphthalmia can be an end-stage effect of trauma, as seen in this patient who was blinded by severe craniofacial trauma. There is residual bilateral microphthalmia and dystrophic changes within the devitalized eyes on both sides. This condition is sometimes referred to as phthisis bulbi.
Magnetic Resonance
MRI might be less definitive with regard to the bony orbit and other facial skeletal features than CT.
MRI may show thickened sclera. It will be equivalent to CT for identifying associated colobomas and associated intraorbital cysts. MRI will be more definitive than CT to evaluate for associated anomalies of the central nervous system that may range from very gross manifestations of holoprosencephaly to relatively subtle disorders of neuronal migration.
MRI would be the preferred method for screening for associated brain malformations, including atrophy of cerebral tissue (microphthalmia brain atrophy) (Fig. 46.3B) or corpus callosum abnormalities (Fig. 46.3E), once the presence of microphthalmia is established.
Ultrasound
Ultrasound might be used as a preliminary tool to look for a coloboma or associated disorder of the eye or orbit, but it will not be definitive in the latter regard (Fig. 46.7).
Perhaps its more important role is in the prenatal monitoring of orbital and eye development in routine obstetric care and more specifically in families at risk for microphthalmia and the many disorders associated with this condition.
Differential Diagnosis
From Clinical Data
Some acquired diseases can cause the eye to be small. Based on age, exposure history, and other clinical features, acquired conditions that may cause the eye to be small are simply excluded. Such conditions may be as widely divergent as retinoblastoma, retinopathy of prematurity (ROP), post radiation, posttraumatic changes, and ocular toxocariasis.
From Supportive Diagnostic Techniques
Imaging will frequently provide clues to the likely etiology, such as showing a coloboma or other developmental abnormalities, intraocular calcification in Coats disease, and retinoblastoma. These findings are discussed more completely in the several conditions known to be associated with microphthalmia.
Medical Decision Making and Treatment Options
Microphthalmia is a marker for other diseases. The more frequent associations will be with cerebral malformations, other eye and orbital abnormalities, and facial dysplasia and clefts. Many other associations are possible and perhaps critical to the family and child.
Genetic counseling and a search for associated systemic disease may be warranted depending on the clinical context.
Surgical
Surgical considerations with regard to the eye and orbit are usually cosmetic. There are methods to support orbital growth in the absence of a growing eye that will lead to better cosmetic outcomes. Occasionally, related congenital cysts will need to be removed.
DISEASE ACUITY AND REPORTING RESPONSIBILITY
These are not acute problems. Initially, proper reporting requires establishing the diagnosis and excluding the possibility that this is an acquired disease. If an unexpected acquired disease such as retinoblastoma or parasitic infection is discovered, direct verbal communication with documentation of that contact would be wise.
Once the likely congenital nature is established, it is necessary to search for associated abnormalities. A complete report should include language indicating that there had been a diligent search for associated abnormalities of the involved orbit and eye as well as the contralateral orbit and eye at minimum.
If other parts of the face or skull brain are imaged coincidentally, one must comment on the possible presence of dysmorphic features or specific abnormalities involving the anatomy on the images.
Suggestions for further evaluation should include the fact that associated abnormalities of the face, brain, temporal bone, and other organ systems and syndromic associations are possible.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree