(1)
Research Institute for Environmental Management Technology, National Institute of Advanced Industrial Science and Technology (AIST), Tsukuba, Japan
Abstract
The plasma membrane of living cells is an interface of material transfers and an antenna for outer signals. This chapter provides a guide on how to fabricate bioluminescent capsules for illuminating intracellular signaling and cargo protein delivery. The capsule consists of four components, which are, in consecutive order: a secretion peptide (SP), a host luciferase body (leader), a guest protein or peptide (cargo), and a membrane localization signal (MLS). Any guest protein, including a luciferase or a fluorescent protein, may be sandwiched between the host luciferase body and MLS and may be deliverable to the plasma membrane (PM), where the capsule waits for outer signals and to quickly release the embedded luciferase in response to a specific signal. The present strategy provides an efficient molecular vehicle for cargo proteins and imaging of intracellular molecular events in living cells without substrate-derived demerits of luciferases.
Key words
ApoptosisBioluminescent capsuleBioluminescenceLuciferaseMembrane localizationImagingSubstrate1 Introduction
Although bioluminescence is an emerging optical readout, it requires excess substrate and molecular oxygen (O2) as a prerequisite, which hampers its use in living cells and complex organisms [1]. In contrast to recent achievements in optical intensity [2, 3], the substrate-derived problems of luciferases, e.g., the membrane permeability of the substrates and the biased diffusion of the substrate into the intracellular compartments of mammalian cells, remain a major drawback in live-cell imaging [4]. In particular, organelle-sequestered luciferases should immediately consume nearby coelenterazine (CTZ) and O2 and may fall into substrate anoxia in living mammalian cells or tissues. Under a deficiency of substrates and O2, the optical intensities should be dominated simply by the rates of the substrate and O2 supply.
To address the substrate-derived problems, the author took note of the privileges of the PM. The PM defines the cell boundaries and acts as a key interface that senses external signals, allowing the cells to change their behavior in response to environmental cues [5]. The PM has particularly distinct privileges over other cellular ingredients on live-cell imaging; e.g., (1) a bioluminescent indicator on the PM immediately responds even to poor membrane-permeable stimulators, (2) infinite amounts of substrates and O2 should be supplied to the probe, and (3) bioluminescence imaging (BLI) is easy on the PM because the entire cell territory is bright and the long-term optical intensity on the PM is stable.
The present chapter provides a detailed guide on how to create a bioluminescent capsule located in the PM, where the capsule waits for outer signals and signal-dependently provokes the release of the embedded luciferase. The capsule consists of a secretion peptide (SP), a host luciferase body a guest protein or peptide (cargo), and a membrane localization signal (MLS) (Fig. 1a). Any guest proteins with specific functionalities may be deliverable by the capsule to the endoplasmic reticulum (ER) and PM. We exemplify the visualization of an apoptosis signaling with the capsule, which carries caspase substrate peptides comprising Asp-Glu-Val-Asp (DEVD) or Ile-Glu-Thr-Asp (IETD) sequences (Fig. 1b). Not only the peptides but also the fluorescent proteins and luciferases were found to be efficiently portable by the capsule.
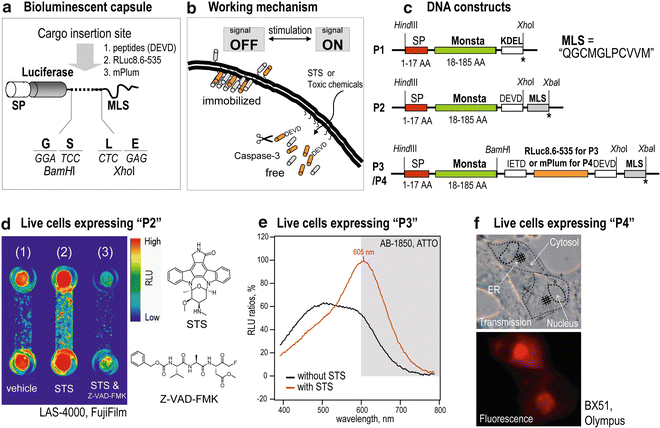

Fig. 1
Live-cell imaging with bioluminescent capsules. (a) A bioluminescent capsule. A luciferase leader (host) is located at the head and followed by a guest protein. The capsule ends with the membrane localization signal (MLS). (b) The working mechanism of a bioluminescent capsule. In the presence of apoptosis signaling, the hinge regions of the capsules are dissected by intrinsic caspases. (c) cDNA constructs encoding bioluminescent capsules. (d) Visualization of apoptosis signaling with a bioluminescent capsule in living cells. (e) Dramatic convergence of the bioluminescence spectra from living cells carrying a bioluminescent capsule according to apoptosis signals. (f) Fluorescence image of live cells carrying a bioluminescent capsule. The plasma membrane (PM) and the endoplasmic reticulum (ER) are illuminated by the capsule. Reproduced from Kim et al. 2012 with permission from Bioconjugate Chemistry (ACS) [4]
2 Materials
2.1 Construction of Bioluminescent Capsules
3.
cDNA encoding fluorescent protein “mPlum” (Clontech).
4.
A mammalian expression vector, pcDNA3.1(+) (Invitrogen).
5.
Custom-made primers for polymerase chain reaction (PCR).
6.
A ligation kit with a ligase, Restriction enzymes (HindIII, BamHI, XhoI), and DNA polymerase.
2.2 Live-Cell Imaging with Bioluminescent Capsules
1.
Native coelenterazine (nCTZ) as the substrate of Monsta and RLuc8.6-535 in a luciferase assay kit (Promega; cat. E2820).
2.
A CTZ derivative, ViviRen® (Promega).
3.
Microslides for cell culture and live-cell imaging (2.5 × 7.5 cm, μ-slide VI0.4; cat. 80606; ibidi, Germany).
4.
Hanks’ balanced salt solution (HBSS) buffer, pH 7.4.
5.
TransIT-LT1® (Mirus) as a lipofection reagent.
6.
Staurosporine (STS) as an apoptosis inducer.
7.
Carbobenzoxy-valyl-alanyl-aspartyl-[o-methyl]-fluoromethylketone (Z-VAD-FMK) as an apoptosis inhibitor.
8.
Dulbecco’s modified Eagle’s medium (DMEM) for cell culture, which is supplemented with 10 % fetal bovine serum (FBS) and 1 % of a mixture of penicillin and streptomycin (P/S).
9.
African green monkey fibroblast-derived COS-7 cells as a mammalian cell. For all the experiments, culture the COS-7 cells in DMEM supplemented with 10 % FBS and 1 % P/S.
10.
Dimethyl sulfoxide (DMSO).
11.
A serum-free medium, e.g., Opti-MEM I®.
12.
Potassium iodide (KI), an additive for boosting bioluminescence intensity (final concentration: 50 mM).
2.3 Instrumentation
1.
GenomeLab GeXP (BeckMan Coulter) as a DNA sequencer.
2.
Tpersonal (Biometra) as a thermal cycler.
3.
LAS-4000 (Fujifilm) as an image analyzer, equipped with a specific image analysis software (Multi Gauge 3.1; Fujifilm).
4.
AB-1850 (ATTO) as a charge-coupled device (CCD) spectrophotometer for accurate light collection in the red region [9].
5.
BX51 (Olympus) as a fluorescent microscope, equipped with a specific control software (AxioVision v4.6).
3 Methods
Carry out all the live-cell imaging procedures at room temperature.
3.1 Creation of a Mammalian Expression Vector Encoding a Bioluminescent Capsule
1.
Generate cDNA encoding a host luciferase, Monsta (1–185 AA; see Notes 1 and 2), by polymerase chain reaction (PCR) with corresponding forward and reverse primers (see Note 3) and templates to introduce unique restriction sites (Hind




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree