Fig. 2.1
Potential pathways and mechanisms for cigarette smoking-mediated cardiovascular dysfunction. The bold boxes and arrows in the flow diagram represent the probable central mechanisms in the complex pathophysiology of cigarette-smoking-mediated atherosclerotic disease. H 2 O 2 hydrogen peroxide, METC mitochondrial electron transport chain, NADPH nicotinamide adenine dinucleotide phosphate reduced form, NOS nitric oxide synthase, ONOO – peroxinitrite, O 2 – superoxide (Reproduced with permission of Elsevier from Ambrose and Barua [8])
Cigarette smoking is a reversible risk factor, such that cessation of smoking leads to a decreased incidence of cardiovascular events as compared to those who do not stop:
Cardiovascular risk declines beginning as early as within 6 months of smoking cessation.
In a large review with over 10,000 patients, results showed a 36 % reduction in crude relative risk of mortality for patients with CHD who quit compared with those who continued smoking [9].
Hypertension
Hypertension is a well-established risk factor for adverse cardiovascular events. High blood pressure is associated with morphologic alterations of the arterial intima and functional alterations of the endothelium that are similar to the changes observed in dyslipidemia and established atherosclerosis:
Data from observational studies involving more than one million individuals have indicated that death from ischemic heart disease and stroke increases progressively and linearly with increases in blood pressure [10].
Both systolic and diastolic blood pressures contribute to increased risk for cardiovascular events (Fig. 2.2).
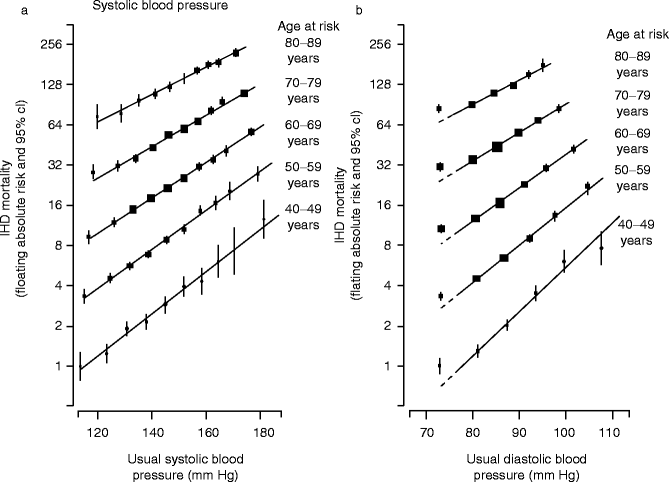
Fig. 2.2
Ischemic heart disease (IHD) mortality rate in each decade of age versus usual blood pressure at the start of that decade. (a) Systolic blood pressure and (b) diastolic blood pressure (Reproduced with permission from Lewington et al. [10])
For every increase of 20 mmHg systolic or 10 mmHg diastolic blood pressure, there is an approximate doubling of mortality from ischemic heart disease and stroke.
The increase in mortality begins at blood pressure levels above 115/75 mmHg.
Based on a comprehensive analysis of 354 randomized trials, treating hypertension with multiple drug therapy was estimated to lower blood pressure by 20 mmHg systolic and 11 mmHg diastolic, thereby reducing the risk of stroke by 63 % and ischemic heart disease events by 46 % [11].
Given the additional data on lifetime risk of hypertension and increased risk of cardiovascular complications associated with BP previously considered to be normal, the JNC 7 report reclassifies 120–139 mmHg SBP and 80–89 mmHg DBP as “prehypertension” [12] (Table 2.1).
Table 2.1
Classification of blood pressure for adults
Blood pressure classification
SBP mmHg
DBP mmHg
Normal
<120
And <80
Prehypertension
120–139
Or 80–89
Stage 1 Hypertension
140–159
Or 90–99
Stage 2 Hypertension
≥160
Or ≥100
The goal of the new classification scheme is to intensify early intervention in order to reduce lifetime risk.
Diabetes Mellitus
On the basis of data from NHANES 2005–2008, an estimated 18,300,000 Americans ≥20 years of age have physician-diagnosed DM (Fig. 2.3). Additionally, 7,100,000 adults have undiagnosed DM and approximately 81,500,000 adults have prediabetes (e.g., fasting blood glucose of 100–126 mg/dL). The prevalence of prediabetes in the US adult population is nearly 37 % [13].
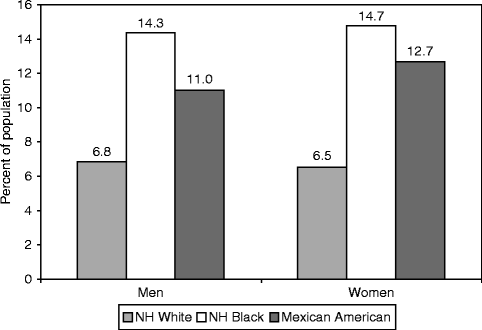
Fig. 2.3
Age-adjusted prevalence of physician diagnosed type 2 diabetes mellitus in adults ≥20 years of age by race/ethnicity (National Health and Nutrition Examination Survey: 2005–2008). NH indicates non-Hispanic (Reproduced with permission from Roger et al. [13])
Hyperglycemia and insulin resistance are components of diabetes that comprise increased risk for atherosclerotic disease [14].
Compared to unaffected individuals, diabetic patients have a greater atherosclerosis burden in both the macrovascular and microvascular circulation.
In a Finnish population-based study, the 7-year incidence of MI in diabetic patients without prior MI was found to be essentially the same as non-diabetic patients with prior MI [15].
There is overwhelming evidence of DM as a significant risk factor for atherosclerosis and that aggressive control of DM can lead to decreased mortality.
A meta-analysis of 37 studies found the rate of fatal coronary heart disease was higher in patients with diabetes than in those without (5.4 % vs. 1.6 %). The relative risk for fatal coronary heart disease in patients with diabetes compared with no diabetes was significantly greater among women than it was among men: 3.50 % vs. 2.06 % [16] (Fig. 2.4).
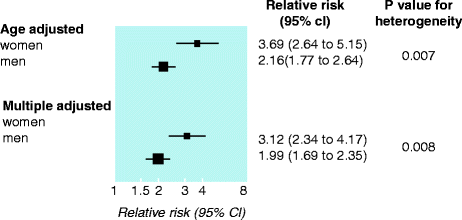
Fig. 2.4
Overall summary estimates of relative risks and 95 % confidence intervals for fatal coronary heart disease in men and women with and without diabetes in 22 studies that reported both age and multiple adjusted coefficients (Reproduced with permission from Huxley et al. [16])
The NECP/ATP III guidelines for the management of cholesterol elevated DM to the highest risk category as a CHD equivalent [17] (Table 2.2).
Table 2.2
Coronary heart disease risk equivalents.
Other forms of clinical atherosclerotic disease
Peripheral arterial disease
Carotid artery disease
Abdominal aortic aneurysm
Diabetes mellitus
Cholesterol lowering with the HMG-CoA reductase inhibitors has yielded important reductions in CHD events in patients with diabetes mellitus.
Dyslipidemia
There are many lipid abnormalities that are associated with increase cardiovascular risk. These include elevated total and LDL cholesterol, low HDL cholesterol, and elevated triglycerides.
The Framingham Heart Study and the Multiple Risk Factor Intervention Trial found a direct and linear relationship between levels of LDL cholesterol (or total cholesterol) and the rate of new-onset CHD in men and women who were initially free of CHD [3, 18]:
Convincing evidence exists that lowering serum cholesterol reduces the risk of subsequent cardiovascular events and overall mortality.
HMG-CoA reductase inhibitors, known as statins, inhibit the rate-limiting step of cholesterol synthesis in the liver.
Statin medications are effective in lowering the serum total cholesterol, LDL cholesterol, and triglyceride levels and, to a smaller degree, in raising the serum HDL cholesterol level.
The collection of large clinical trials of the HMG-CoA reductase inhibitors has substantiated the decrease in total mortality in study populations.
As an example in the primary prevention of coronary artery disease:
The Air Force/Texas Coronary Atherosclerosis Prevention Study (AFCAPS/TexCAPS) examined the effects of lovastatin on the incidence of a first major coronary event in 5,608 men and 997 women with average total cholesterol and LDL cholesterol and below average HDL cholesterol levels.
A statistically significant 37 % reduction in the incidence of the first major coronary event (4 % vs. 6.8 %) occurred after an average of 5.2 years [19].
The Cholesterol Treatment Trialists’ Collaborators performed a prospective meta-analysis of data from over 90,000 individuals in 14 randomized trials of statins and demonstrated a linear decrease in clinical outcomes per 1.0 mmol/L (39 mg/dL) reduction in LDL cholesterol [20] (Fig. 2.5).
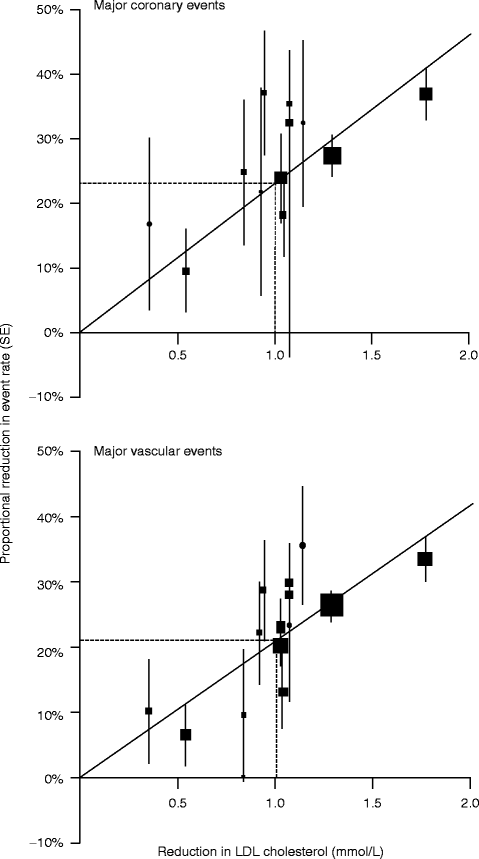
Fig. 2.5
Relation between proportional reduction in incidence of major coronary events and major vascular events and mean absolute LDL cholesterol reduction at 1 year. Square represents a single trial plotted against mean absolute LDL cholesterol reduction at 1 year, with vertical lines above and below corresponding to one SE of unweighted event rate reduction. Trials are plotted in order of magnitude of difference in LDL cholesterol difference at 1 year. For each outcome, regression line (which is forced to pass through the origin) represents weighted event rate reduction per mmol/L LDL cholesterol reduction (Reproduced with permission from Cholesterol Treatment Trialists’ Collaborators [20])
Strong epidemiological evidence links low levels of serum HDL cholesterol to increased CHD morbidity and mortality. High HDL-cholesterol levels are a protective risk factor against CHD (Table 2.3).
Table 2.3
Risk factors that modify the LDL goal per ATPIII guidelines
Cigarette smoking
Family history of premature CHD (CHD in male first degree relative <55 years; CHD in female first degree relative <65 years)
Age (Men ≥ 45 years, women ≥ 55 years)
Hypertension (BP > 140/90 mmHg or on antihypertensive medication)
Low HDL cholesterol (<40 mg/dL)
“Negative” risk factor high HDL cholesterol (>60 mg/dL), its presence removes one risk factor.
Each 1 mg/dL reduction in HDL cholesterol is associated with a 2–3 % increase in relative risk for CHD [21].
Nicotinic acid effectively increases HDL cholesterol levels in a dose-dependent manner related in part to stimulation of production of apoprotein A1, the principal apoprotein of HDL.
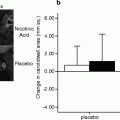
Treatment with nicotinic acid has been shown to decrease the incidence of recurrent CHD when compared to placebo and convincingly lead to atherosclerosis regression [22] (Fig. 2.6).
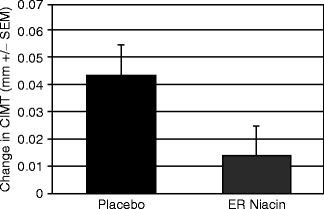
Fig. 2.6
Serial mean CIMT measurements from the far wall of the bilateral common carotid arteries at baseline and 12 months during treatment with either placebo or extended-release niacin (Niaspan) added to stable statin therapy. Progression rates in the 2 groups were 0.044 ± 0.011 mm (placebo; P = 0.001) and 0.014 ± 0.011 mm (Niaspan, P = 0.23) (Reproduced with permission from Taylor et al. [22])
Although low HDL cholesterol is known to be a risk factor for CHD and treatment with medications to increase HDL leads to atherosclerosis regression, the large federally funded AIM-HIGH (Atherothrombosis Intervention in Metabolic Syndrome with Low HDL/High Triglycerides) trial was stopped early because the addition of niacin to a statin did not reduce the risk of cardiovascular events [23]. Due to a number of important trial design limitations and the limited power and duration of the trial, definitive understanding of the impact of niacin on clinical cardiovascular outcomes awaits further investigation.
Elevated serum triglycerides are associated independently with an increased risk of CHD. Furthermore, elevated triglycerides are also associated with other risk factors such as low HDL, elevated LDL, and obesity [24].
Triglyceride rich lipoproteins can be summated using calculation of “non-HDL” cholesterol (total cholesterol – HDL). Therapeutic goals for non-HDL are 30 mg/dL above those for LDL cholesterol as specified by the NCEP-ATPIII.
Treatment of TG elevations can include therapeutic lifestyle changes, fibrates, and nicotinic acid [17]. Gemfibrozil monotherapy has been demonstrated to reduce cardiovascular events in both primary and secondary prevention settings when dyslipidemia is present.
Therapy with lipid lowering agents should be a component of multiple risk factor intervention and is only indicated as an adjunct to diet therapy when the response to a diet restricted in saturated fats and cholesterol has been inadequate. The NCEP guidelines recommend aggressive lipid-lowering therapy for patients at high risk for coronary heart disease [17].
Metabolic Syndrome
This syndrome is characterized by multiple interrelated metabolic factors in one individual that increase risk for atherosclerosis.
US survey data from the National Health and Nutrition Evaluation show that the metabolic syndrome is found in one in four adults, with an increasing prevalence with age.
The components of metabolic syndrome are as follows:
Abdominal obesity: waist circumference:
Men > 40 in. (>102 cm)
Women > 35 in. (>88 cm)
Asian populations have lower waist circumference thresholds (90 and 80 cm respectively for men and women)
Fasting glucose ≥ 110 mg/dL
Triglycerides ≥ 150 mg/dL
Blood pressure ≥ 130/85 mmHg
HDL cholesterol:
Men < 40 mg/dL
Women < 50 mg/dL
The metabolic syndrome is mechanistically associated with a generalized metabolic disorder called insulin resistance, in which tissue responsiveness to the normal action of insulin is impaired.
The metabolic syndrome is most important because of its association with the subsequent development of Type II diabetes mellitus and cardiovascular disease.
The pathogenesis of the syndrome has multiple origins, but obesity and sedentary lifestyle coupled with diet and still largely unknown genetic factors clearly interact to produce the syndrome.
In a large Finnish prospective population-based study, the presence of metabolic syndrome increased cardiovascular mortality by two to fourfold [25].
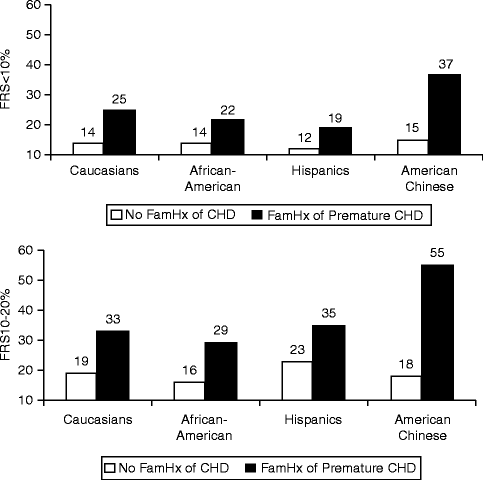
Family History
Family history of premature CHD (onset age <55 years in father, <65 years in mother) is a significant independent risk factor for cardiovascular disease.
Analysis from the Framingham Offspring Study in an 8-year follow up showed that those patients with at least one parent with premature cardiovascular disease had a significantly increased risk for a cardiovascular event (odds ratio 2.0 for men and 1.7 for women) [26].
In the Multi-ethnic Study of Atherosclerosis (MESA), patients of multiple ethnicities with a family history of premature CHD had significantly increased prevalence of advanced subclinical coronary artery disease, defined as an Agatston coronary artery calcium score ≥75th percentile for age, gender and ethnicity (Fig. 2.7).