Fair Game
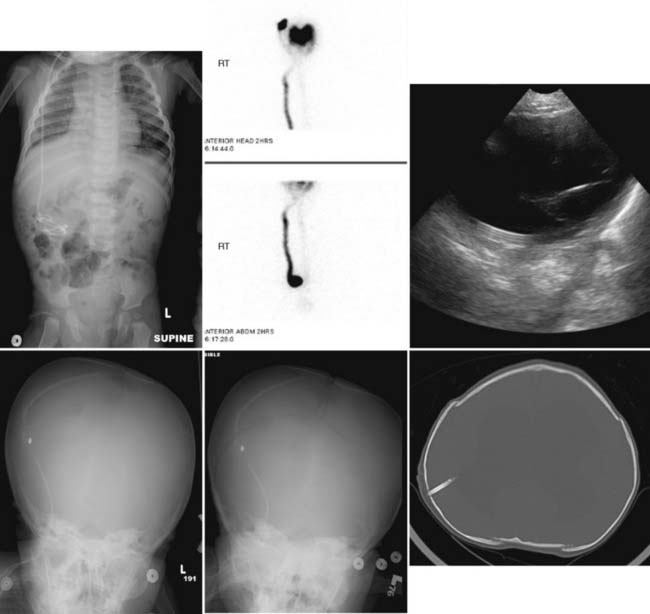
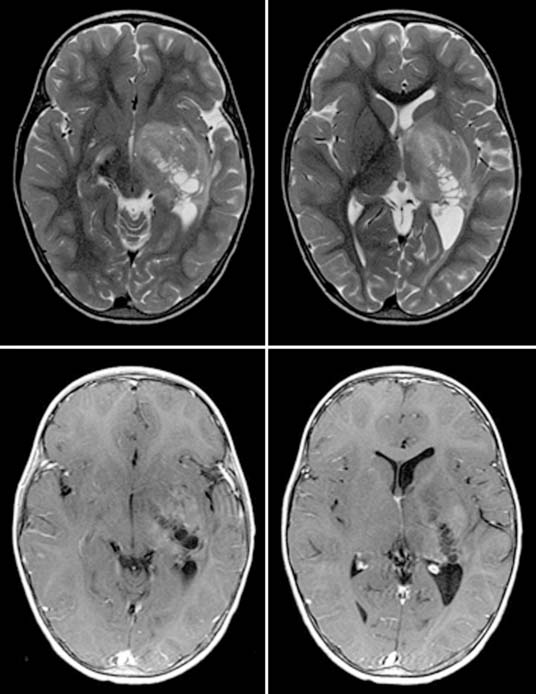
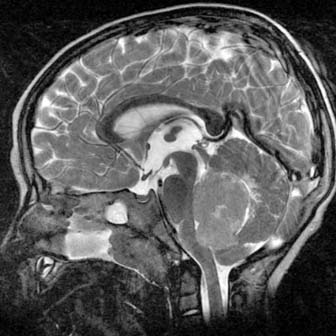
Case 49
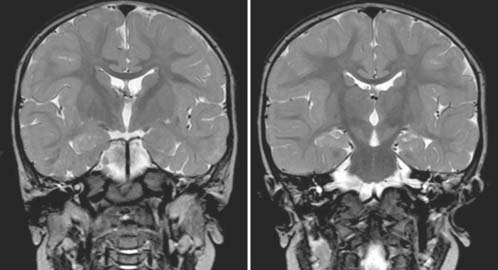
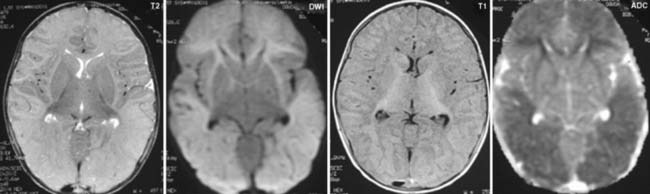
Answers: Case 49
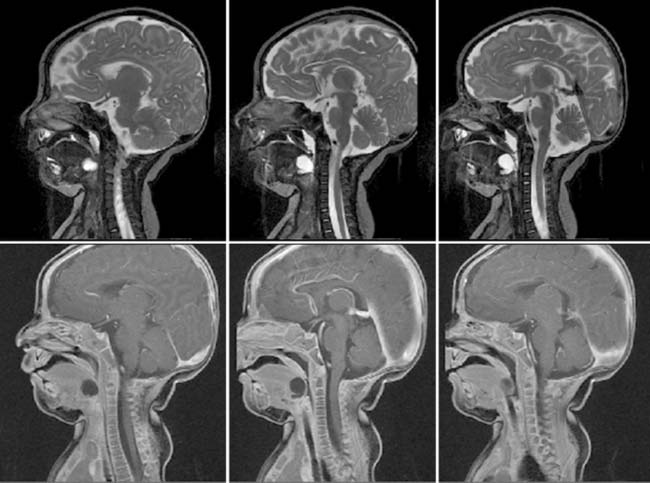
Diagnosis: Ventriculoperitoneal Shunt Complications
Case 51
Case 52
Kayser R., Mahlfeld K., Greulich M., et al. Spondylodiscitis in childhood: results of a long-term study. Spine. 2005;30:318-323.
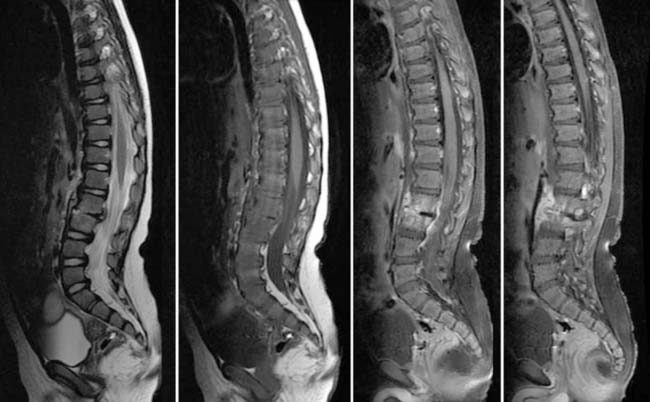
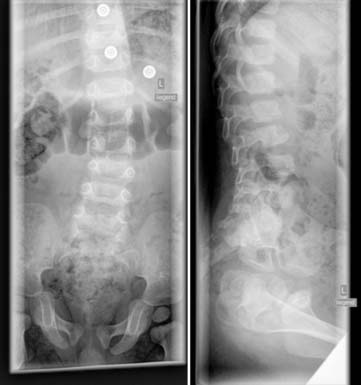
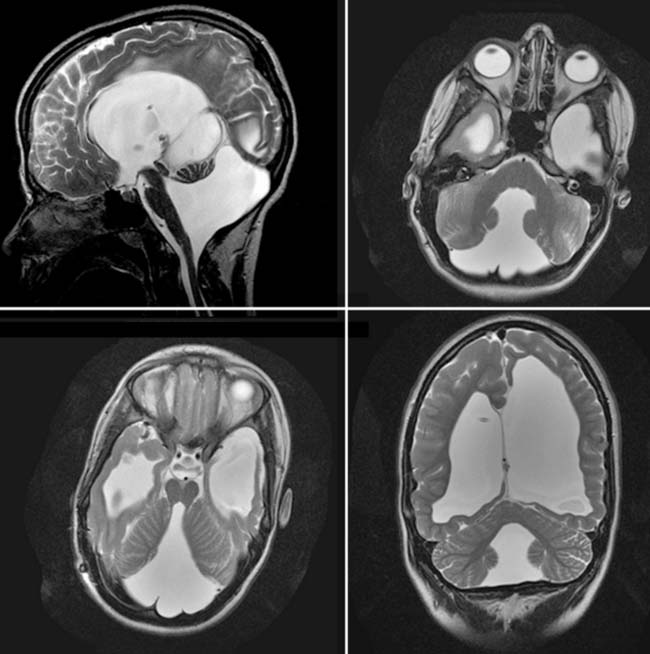
Case 53
Answers: Case 53
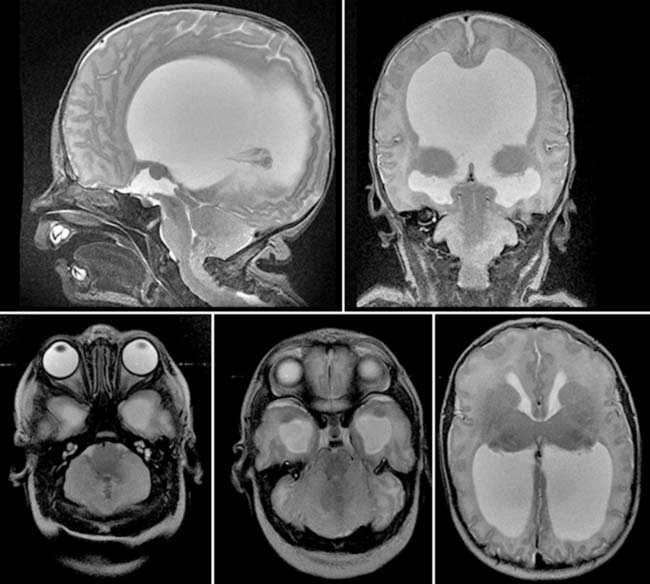
Diagnosis: Arnold-Chiari II Malformation
Barkovich A.J. Pediatric neuroradiology, diagnostic imaging. Salt Lake City: Amirsys Inc, 2007;III 16-III 19.
Case 54
Answers: Case 54
Patel S., Barkovich A.J. Analysis and classification of cerebellar malformations. AJNR Am J Neuroradiol. 2002;23:1074-1087.
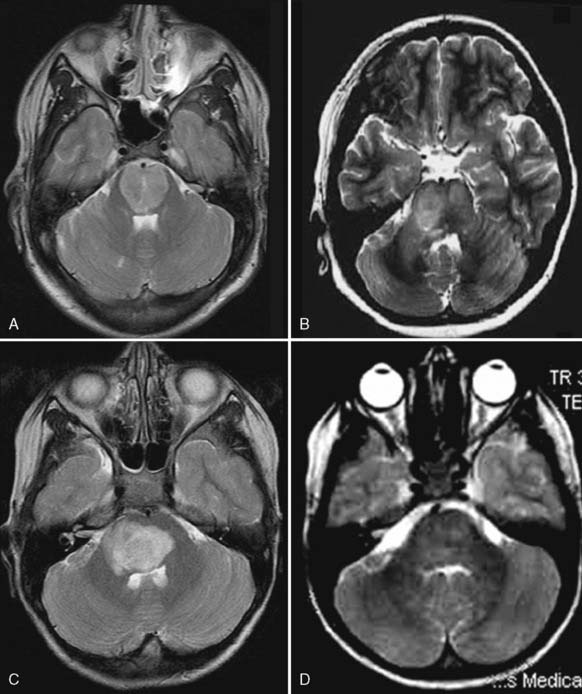
Case 55
Answers: Case 55
Case 56
Answers: Case 56
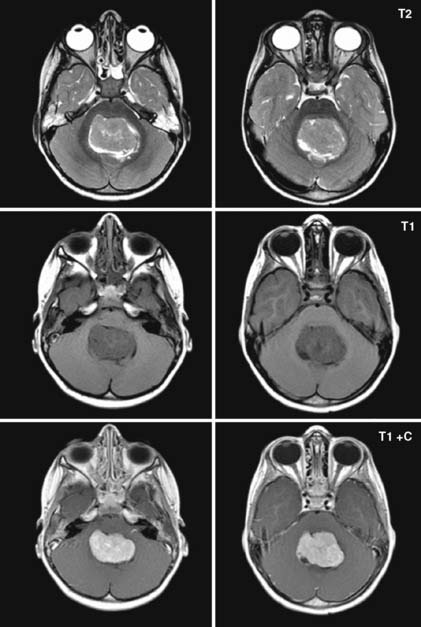
Case 57
Answers: Case 57
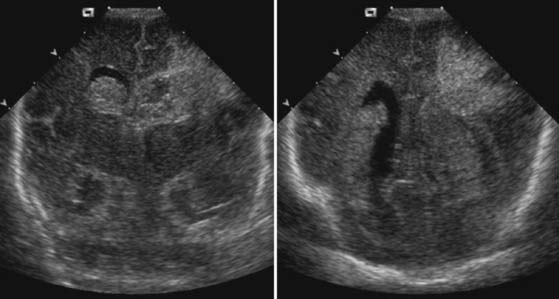
Case 58
Answers: Case 58
Diagnosis: Germinal Matrix Hemorrhage with Venous Infarction
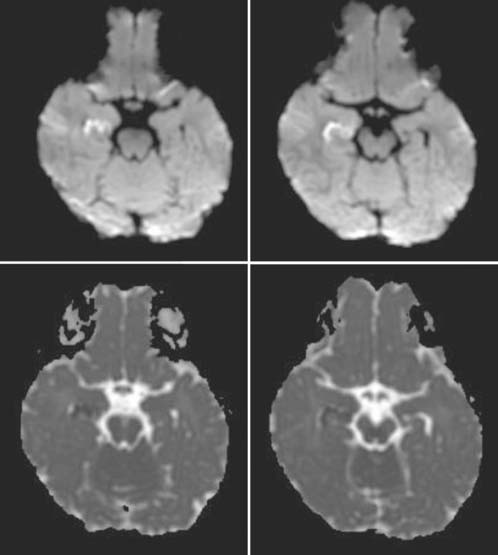
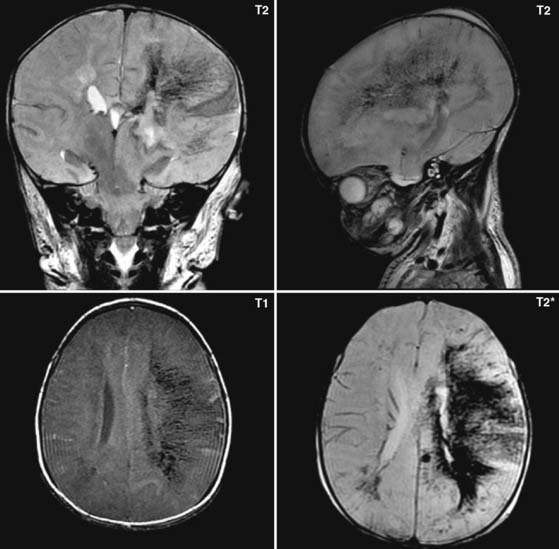
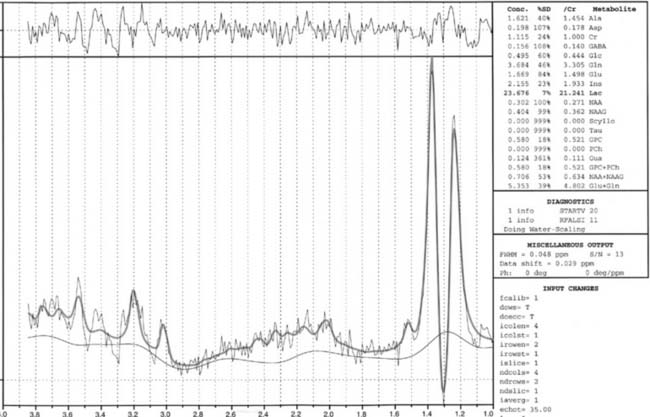
Case 59
Answers: Case 59
Diagnosis: Hypoxic-Ischemic Injury
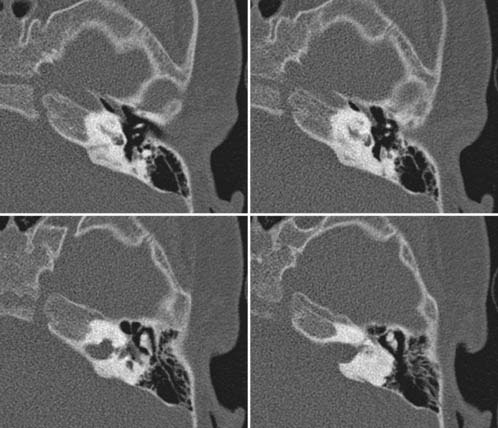
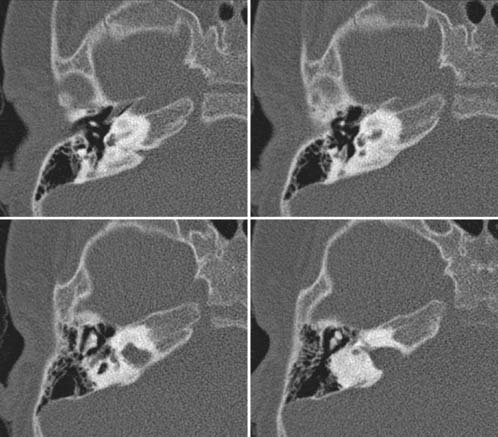
Case 60
Answers: Case 60
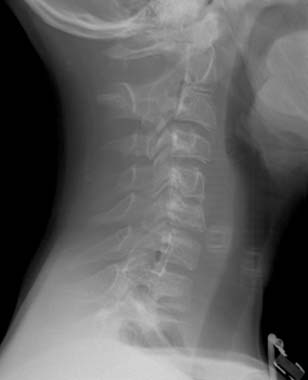
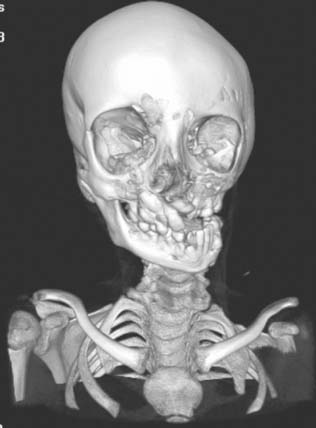
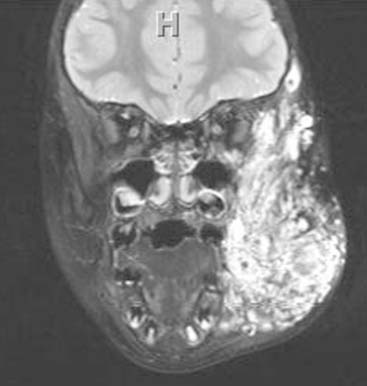
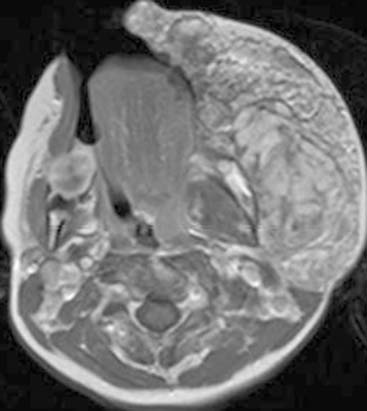
Diagnosis: Goldenhar Syndrome (Goldenhar-Gorlin Syndrome, Facioauriculovertebral Sequence)
Taybi H., Lachman R.S. Radiology of syndromes, metabolic disorders and skeletal dysplasias, ed 4, Baltimore: Mosby; 1996:356-358.
Case 61
Answers: Case 61
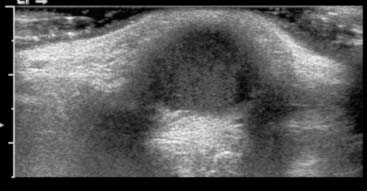
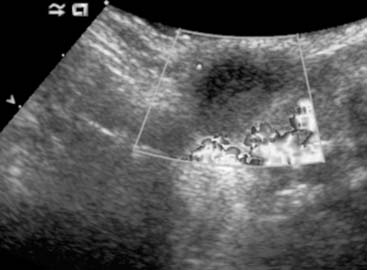
Diagnosis: Thyroglossal Duct Cyst
Moore K.M. The developing human, ed 2, Philadelphia: Saunders; 1977:160-180.
Barkovich AJ, Moore KR, Jones BV, et al, editors: Diagnostic imaging: neuroradiology, Salt Lake City, 2007, Amirsys, II 4:18-21.
Case 62
Answers: Case 62
Arndt C.A.S., Crist W.M. Common musculoskeletal tumor of childhood and adolescence. N Engl J Med. 1999;341:342-352.
Case 63
Answers: Case 63
Diagnosis: Subglottic Stenosis
Tekes A., Flax-Goldenberg R. Diagnostic imaging of the pediatric airway. Operative techniques in otolaryngology. Head Neck Surg. 2007;18(2):115-120.
Case 64
Answers: Case 64
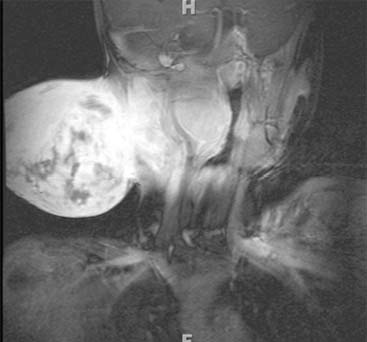
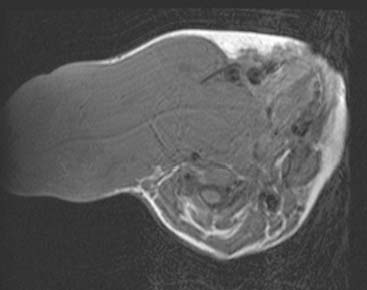
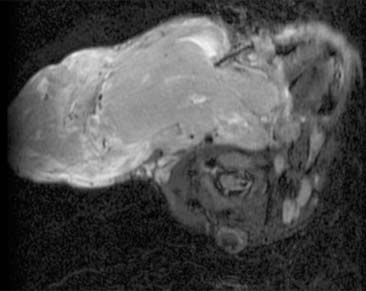
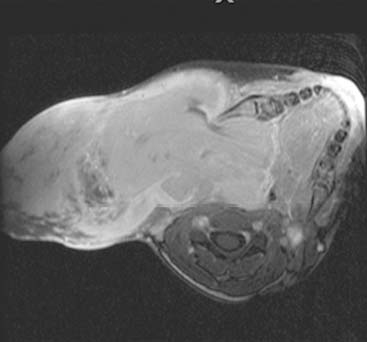
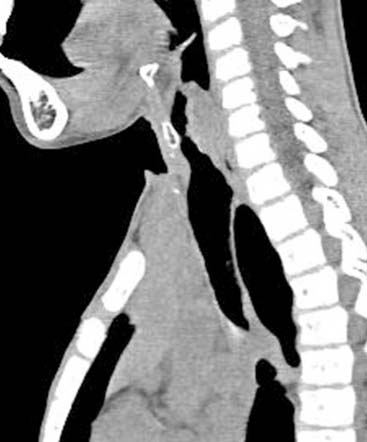
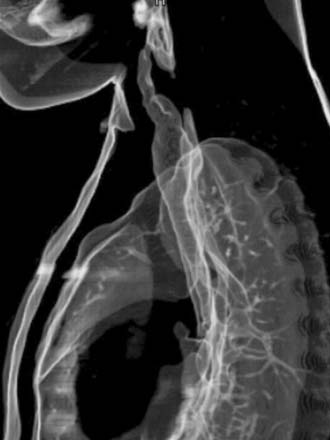
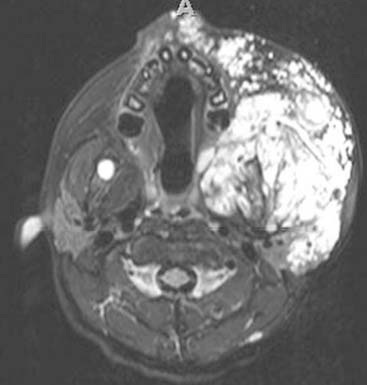
Diagnosis: Venous Malformation
Case 65
Answers: Case 65
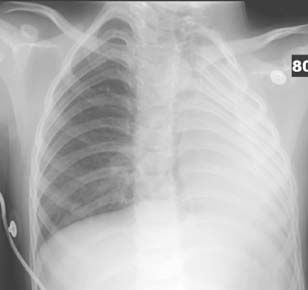
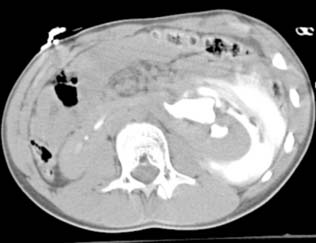
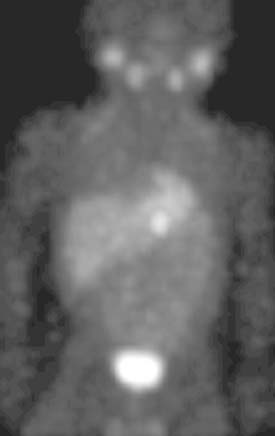
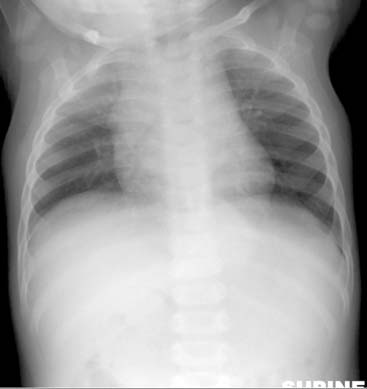
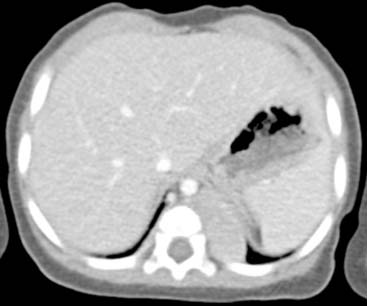
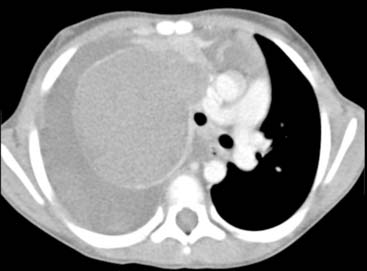
Diagnosis: Paraspinal, Posterior Mediastinal Neuroblastoma
Kuhn J.P., Slovis T.L., Haller J.O. Caffey’s pediatric diagnostic imaging, ed 10, Philadelphia: Mosby; 2004:1210-1215.