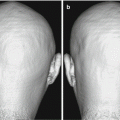
Fig. 9.1
Representative FDG-PET images of AD patient. (a) Tomography, (b) 3D-SSP, and (c) 3D-SSP Z-score images show hypometabolism in the parietotemporal association cortex, posterior cingulate, and precuneus
Reduction in glucose metabolism in the posterior cingulate and precuneus is considered to be an early diagnostic clue for AD [18], because this area lies inside the brain, visual assessment of subtle reductions in metabolism on PET cross-section images is not easy. This may be overcome by the use of statistical image analysis methods, such as the widely used 3D-stereotactic surface projection (3D-SSP) [19] and statistical parametric mapping (SPM) [20]. These are tools which can easily evaluate all the cerebral regions by comparing individual case images with normal database. 3D-SSP applies a z-score mapping to areas with reduced or increased glucose metabolism. Not only does this simplify the assessment of medial side of the brain, it also offers high reproducibility and detection of slight changes.
Many reports have examined the diagnostic ability of FDG-PET on cases that had been diagnosed with AD according to clinical diagnostic criteria such as NINCDS-ADRDA and DSM-IV [19, 21–42]. Reported sensitivity and specificity vary widely, but according to a meta-analysis, the sensitivity and specificity of FDG-PET are 90% and 89%, respectively, demonstrating FDG-PET’s superiority over biomarkers such as cerebrospinal fluid Aβ42 and tau, cerebral blood flow SPECT, and MRI [43]. Furthermore, analysis of the literature published after the year 2000 revealed the extremely high diagnostic ability offered by FDG-PET, with 96% sensitivity and 90% specificity. This improvement in diagnostic ability is thought to be due to the use of improved PET devices or improved interpretational skills of observers [44]. However, the best that FDG-PET can do is differentiating between patients with AD and healthy subjects: it must be noted that it does not incorporate histopathological evidence, and clinical diagnosis is the current gold standard.
9.3 Differentiation between Alzheimer’s and Non-Alzheimer’s Dementia
About half of the patients with dementia have AD, but there are also various cases of non-AD dementia, such as dementia with Lewy bodies (DLB), frontotemporal dementia (FTD), and vascular dementia (VaD), which must be differentiated from AD. Differentiating between non-AD dementia and AD is important for deciding on the treatment approach, estimating future symptoms, and determining prognosis. There are also a number of reports about differentiation between AD and non-AD dementia with FDG-PET [26, 27, 29, 38, 45–52], but according to a meta-analysis, the sensitivity and specificity of FDG-PET are 93% and 70%, respectively; the specificity is somewhat low [43]. Even if only reports with histopathological confirmation are selected, specificity still tends to be low [48, 50–52]. Among non-AD dementia cases, there are many instances of false positive FDG-PET scans which show hypometabolic patterns similar to those seen in AD.
The frequency of DLB is high among non-AD dementia cases. Differential diagnosis of DLB and AD is important for accurate prognostication and appropriate treatment; however, it is not easy because there is clinically overlapping symptoms and hypometabolism observed in DLB is similar to that observed in AD (Fig. 9.2). In addition to a decline in glucose metabolism that is similar to that found in AD, hypometabolism in the occipital lobe including visual cortex is characteristic of DLB. Low uptake in occipital lobe on SPECT and/or PET is one of the supportive features suggested as a clinical diagnostic criterion for DLB [53], although specificity is not high [45–47]. Also, regarding differentiation between AD and FTD, which are covered by the US Medicare system insurance, FDG-PET has been shown to have 99% sensitivity and 65% specificity in a study of a large number of cases [38]. In reports confirmed by histopathological diagnosis, sensitivity was high at 97% and specificity was 71% [48]. These results suggest that it is not uncommon for DLB and FTD to be diagnosed as AD.


Fig. 9.2
Representative FDG-PET images of DLB patient. (a) Tomography, (b) 3D-SSP, and (c) 3D-SSP Z-score images show hypometabolism in the occipital lobe, parietotemporal association cortex, posterior cingulate, and precuneus
9.4 FDG-PET for Mild Cognitive Impairment
MCI was proposed and revised by Petersen et al. as a summary of cognitive function status expressing normal and dementia statuses [54]. Subtypes include amnestic type and non-amnestic type; single-domain types, in which disability forms in the single higher brain function area; and multi-domain type, in which the disability manifests in many areas. The cause of MCI is heterogeneous and involves a number of conditions; besides AD, degenerating dementia, such as DLB and FTD; cerebrovascular disease, including VaD; and psychiatric conditions, such as depression and external injury-type changes; and normal aging [54, 55]. Among these, amnestic-type MCI patients convert to AD at a rate of about 12–15% per year [54]. If disease-modifying treatment is developed in the future, the MCI stage would be considered to be the appropriate period to begin treatment, so there is an especially high necessity for early diagnosis at this stage. Early diagnosis at the MCI stage using FDG-PET allows prediction of conversion from MCI to AD. In past reports with 1–2-year follow-up periods, the accuracy of predicting conversion from MCI to AD was high, at 80% or greater [56–58], and in a meta-analysis conducted by Yuan et al., sensitivity and specificity both tended to be high, at 88.8% and 84.9%, respectively [59]. However, recent reports using ADNI data suggested low diagnostic ability, with a sensitivity of 57% and a specificity of 67% [60]. This lack of consistency in diagnostic ability may be due to differences in backgrounds of the registered MCI groups, discrepancies in analysis or evaluation methods, or differences in follow-up periods.
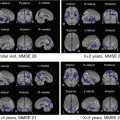
In Japan, a prospective multicenter study targeting amnestic MCI, the Study on Diagnosis of early Alzheimer’s disease-Japan, SEAD-J, was conducted; this report contains the results of 3-yr. observations of progress [61]. According to this study, the diagnostic ability of FDG-PET using visual evaluation has a sensitivity of 98%, specificity of 41%, and an accurate diagnosis rate of 71%. This trend coincides with the results of a similar multicenter study using cerebral blood flow SPECT, the Japan Cooperative SPECT Study on Assessment of Mild Impairment of Cognitive Function, J-COSMIC [62], but the overall diagnostic ability of FGD-PET is higher. In the SEAD-J extension, which included a 5-year follow-up of SEAD-J cases, there were cases of MCI that converted to AD in the fourth or fifth year (Fig. 9.3); hence, it may be possible that the low specificity is caused by being a slow converter. Compared with slow converters, rapid converters have clear tendencies for AD-type changes on baseline images (Fig. 9.3). In fact, on assessments by mathematical indicators using AD t-sum, diagnostic ability was highest at 2 years when sensitivity was 70%, specificity was 90%, and accuracy was 83%, whereas at 3 years, sensitivity was 60%, specificity was 91%, and accuracy was 77% [61]. These results indicate that if there is no hypometabolism that suggests AD, the probability of conversion from MCI to AD is low and that selecting cases with clear-cut AD changes using semi-quantitative indicators could make it possible to select rapid converters up to the second year.


Fig. 9.3
3D-SSP images of MCI patients converted to AD. Top: Baseline image of rapid converter during a visit in the second year of follow-up. (a) 3D-SSP image, (b) 3D-SSP Z-score image. Glucose metabolism decreased in the parietotemporal association cortex, posterior cingulate, and precuneus mainly on the left side. Bottom: Baseline image of slow converter during a visit in the fifth year of follow-up. (c) 3D-SSP image, (d) 3D-SSP Z-score image. Glucose metabolism decreased in the parietotemporal association cortex, posterior cingulate, and precuneus. Rapid converter exhibited clearer AD changes at baseline than slow converter
9.5 Preclinical Stage FDG-PET
There are a limited number of reports on the use of FDG-PET in the asymptomatic preclinical stage. In the middle age and older groups at high risk for AD due to family history and positive ApoE ε4, hypometabolism of the parietotemporal association cortex has been reported [63, 64]; this abnormality is also seen in their 20s and 30s [65]. In asymptomatic middle aged and older cases with positive ApoE ε4, parietotemporal association cortex and posterior cingulate glucose metabolism declines at an annual rate of 2% [66]. In elderly cases with normal cognitive function progressing to MCI within 3 years of observation, abnormalities were found in the hippocampus, suggesting that abnormal glucose metabolism may start in this area [67]. It is difficult to predict progression to MCI or AD on an individual level because changes in the association cortex and inner part of the temporal lobe during the preclinical stage are normally extremely subtle; but in cases of preventive intervention for asymptomatic cases, FDG-PET may determine efficacy of medical and non-pharmacologic treatment.
In recent years, a substantial number of cognitively normal, elderly individuals without proven deposition of Aβ on amyloid imaging were reported to have at least one significant marker of neurodegeneration including FDG metabolism [68–70]. Cases like these are placed in a separate category from preclinical AD and are designated as “suspected non-AD pathology, SNAP.” It may be affected by the presence of conditions such as cerebrovascular disease, tauopathy, or synucleinopathy, but further verification is required.
References
1.
Hertz E, Dienel GA. Energy metabolism in the brain. In: Dwyer DS, editor. Glucose metabolism in the brain. International review of neurobiology, vol. 51. San Diego, CA: Academic; 2002. p. 2–102.
2.
3.
4.
Liu X, Erikson C, Brun A. Cortical synaptic changes and gliosis in normal aging, Alzheimer’s disease and frontal lobe degeneration. Dementia. 1996;7(3):128–34.PubMed
5.
Weiner MW, Veitch DP, Aisen PS, et al. The Alzheimer’s disease neuroimaging initiative: a review of papers published since its inception. Alzheimers Dement. 2013;9(5):111–94.Crossref
6.
7.
McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):263–9.CrossrefPubMedPubMedCentral
8.
Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):270–9.CrossrefPubMedPubMedCentral
9.
Sperling RA, Aisen PS, Beckett LA, et al. Toward defining th preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):280–92.CrossrefPubMedPubMedCentral
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree