Female Pelvis Ultrasound
Sonography plays the primary role in imaging of the female pelvis. CT and MR are supplemental techniques used when the US examination is equivocal and in the staging of pelvic malignancy. Primary indications for female pelvic US examination are pelvic pain, abnormal vaginal bleeding, and suspicion of pelvic mass. Additional indications include evaluation of precocious puberty, infertility, and early cancer detection.
Imaging Technique
US examination of the pelvis is routinely performed both transabdominally with a full bladder and transvaginally with the bladder empty. Additional examination techniques include the translabial imaging and sonohysterography.
Examination is often begun using the transabdominal (TA) approach. The patient is asked to fill her bladder by drinking several glasses of fluid and by not urinating for at least 1 hour prior to examination. The patient lies supine on the examination table. A 3.5-5.0-MHz sector transducer is placed on the lower abdomen just above the symphysis pubis and the pelvic organs are examined through the window of the distended bladder. Bladder filling is ideal when the bladder dome is just above the uterine fundus. Overdistention compresses the normal anatomy and displaces masses and fluid out of the pelvis. Underdistention limits visualization. Images are obtained in sagittal and transverse planes. To optimally image the uterus, the transducer is aligned with the long axis of the uterus, which is often angled right or left from the midline cervix. The ovaries and adnexa are often best seen by sliding the transducer to the contralateral side and angling back toward the ovary of interest. Measurements are obtained of the uterus, ovaries, and any masses detected in three orthogonal planes. Volumes may be calculated using the formula: volume = length × width × height × 0.52. The TA technique provides the best overview of the pelvis, and is best for examining large masses, but is less comfortable for the patient because of the distended bladder.
For transvaginal (TV) examination, the patient is asked to empty her bladder and lie supine with her legs flexed. Folded sheets or pads are placed under her buttocks to elevate her pelvis above the examination table to allow room for transducer manipulation. Alternatively, the patient may be examined on a pelvic examination table with her feet in stirrups.
A 4.0-7.0-MHz TV US probe is coated with gel and is covered with a sterile condom that is also coated with gel. The probe may be placed in the vagina by the patient, the sonographer, or the sonologist. Prudence dictates that a woman should be in the room at all times during a vaginal sonogram, either as the examiner or as a chaperone [1]. The uterus is examined for size, shape, contour, orientation, and appearance of the myometrium, endometrium, and cervix. The ovaries are documented for size, shape, contour, echogenicity, and position. Any masses or abnormalities are evaluated for origin, echotexture, size, shape, and relationship to other organs. The cul-de-sac is examined for fluid and masses. Loops of bowel in the pelvis are inspected for peristalsis and wall thickening.
A 4.0-7.0-MHz TV US probe is coated with gel and is covered with a sterile condom that is also coated with gel. The probe may be placed in the vagina by the patient, the sonographer, or the sonologist. Prudence dictates that a woman should be in the room at all times during a vaginal sonogram, either as the examiner or as a chaperone [1]. The uterus is examined for size, shape, contour, orientation, and appearance of the myometrium, endometrium, and cervix. The ovaries are documented for size, shape, contour, echogenicity, and position. Any masses or abnormalities are evaluated for origin, echotexture, size, shape, and relationship to other organs. The cul-de-sac is examined for fluid and masses. Loops of bowel in the pelvis are inspected for peristalsis and wall thickening.
The TV technique offers better tissue characterization because of the ability to use high-frequency transducers. However, the field of view is limited and large masses may be overlooked. If TV examination is performed first, the pelvis should always be examined transabdominally, even if the bladder is empty, to check for abnormalities outside the TV field of view.
The translabial approach is particularly useful for examination of the vagina and cervix, and is an effective alternative for any patient unwilling or unable to undergo TV examination [2]. A 3.5-5.0-MHz sector transducer, covered with a transducer sheath and generously coated with gel, is placed on the labia. Examination is performed in sagittal and coronal planes along the long axis of the vagina.
A transrectal approach to pelvic US may also be used especially in patients unwilling or unable to undergo TV US. The transrectal approach offers similar advantages of placing the transducer closer to the organs of interest allowing higher frequency transducers with better resolution.
Sonohysterography (SHG) is a useful technique to examine the endometrium and uterine cavity [3, 4, 5]. The examination is easiest to perform with the patient on a pelvic examination table with her feet in stirrups. A speculum is used to visualize and cleanse the cervix with antiseptic solution. A 5-F pediatric feeding tube or a balloon SHG catheter, pre-filled with sterile saline solution, is placed into the cervix. If a balloon catheter is used, the balloon is distended in the cervical canal with 3-5 mL of water, not air. The speculum is removed and a TV US transducer is placed into the vagina. Using direct US visualization, saline is injected to distend the uterine cavity, detect polypoid masses, and to assess the appearance and thickness of the endometrium.
Anatomy
The size and shape of the uterus vary with age and parity. The uterus of the neonate, stimulated by maternal hormones as a fetus, is up to 1 cm longer and 1 cm larger in diameter than the uterus of a young child. The cervix is commonly twice as long and twice as thick as the body. By the end of the first year of life the uterus has become smaller and is sausage shaped. With puberty, the uterus assumes a pear shape with the body twice as large as the cervix. The uterus enlarges with multiparity and atrophies following menopause. Normal uterine dimensions are listed in Table 5.1.
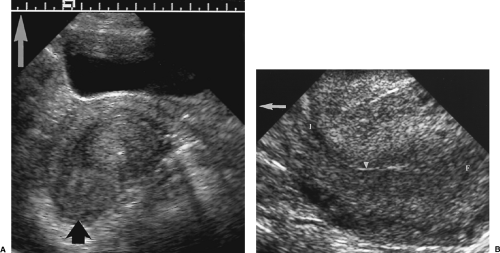
The surface of the uterus is smooth and well defined (Fig. 5.1). A slightly indented isthmus separates the body from the cervix. The cervix is fixed in the midline of the pelvis,
but the body is often “flexed” and angled (“verted”) with respect to the cervix. The uterus is most commonly anteflexed and anteverted, lying on the bladder dome. A retroflexed uterus is bent at the isthmus with the body folded backwards on the cervix (Fig. 5.2). A retroverted uterus is straight but directed posteriorly. The body may also be angled toward the right or left pelvic sidewalls.
but the body is often “flexed” and angled (“verted”) with respect to the cervix. The uterus is most commonly anteflexed and anteverted, lying on the bladder dome. A retroflexed uterus is bent at the isthmus with the body folded backwards on the cervix (Fig. 5.2). A retroverted uterus is straight but directed posteriorly. The body may also be angled toward the right or left pelvic sidewalls.
Table 5.1: Normal Dimensions of the Uterus | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
The myometrium is medium in echogenicity and granular in echotexture. Three layers of myometrium can often be recognized. The inner junctional myometrium is thin, compressed, hypovascular, and mildly hypoechoic compared to the thick homogeneous middle layer. The arcuate vessels, which are often prominent, divide the middle layer from the slightly hypoechoic outer layer (Fig. 5.3). Calcification of the arcuate arteries occurs in older women and diabetics (Fig. 5.4).
The endometrium varies in thickness and appearance with the degree of stimulation by hormones, primarily estrogens and progesterones (Table 5.2). In the neonate, because of maternal hormones, the endometrium is brightly echogenic but thin. In the prepubertal child, the endometrium remains thin and is nearly isoechoic with the myometrium. Following puberty, the endometrial appearance varies with the menstrual cycle [6]. In the proliferative phase (Fig. 5.5A), prior to ovulation, the endometrium assumes a three-layer appearance as it thickens to 4-8 mm. The central line, which defines the endometrial cavity, is echogenic. The proliferating functional layer, which will slough with menstruation, is hypoechoic. The outer basal layer, which remains intact throughout the menstrual cycle, is echogenic and surrounded by the hypoechoic junctional zone of the myometrium. In the secretory phase, following ovulation, the functional layer continues to thicken and becomes echogenic (Fig. 5.5B). The entire double-layer thickness of the endometrium in the secretory phase is 7-14 mm. During menstruation, the functional layer is lost and the
remaining basal endometrium appears as a thin, broken, irregular echogenic line. Following menopause, the endometrium atrophies, thins (to <4 mm), and becomes less echogenic. Hormone replacement therapy in the postmenopausal woman will stimulate the endometrium. Unopposed estrogen has the greatest effect. Sequential hormone replacement, with estrogen followed by progesterone, causes the endometrium to change to an appearance very similar to the premenopausal woman.
remaining basal endometrium appears as a thin, broken, irregular echogenic line. Following menopause, the endometrium atrophies, thins (to <4 mm), and becomes less echogenic. Hormone replacement therapy in the postmenopausal woman will stimulate the endometrium. Unopposed estrogen has the greatest effect. Sequential hormone replacement, with estrogen followed by progesterone, causes the endometrium to change to an appearance very similar to the premenopausal woman.
Table 5.2: Normal Endometrial Appearance and Thickness | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||
The fallopian tubes extend laterally from the uterus in the free edge of the broad ligament. Each is 7-12 cm in length and consists of an intramural portion traversing the myometrium, a cord-like isthmus, a wider and tortuous ampullary portion, and the funnel-shaped, fimbriated infundibulum that opens into the peritoneal cavity. The fallopian tubes are not appreciated on US unless they are dilated or when peritoneal fluid defines the edge of the broad ligament.
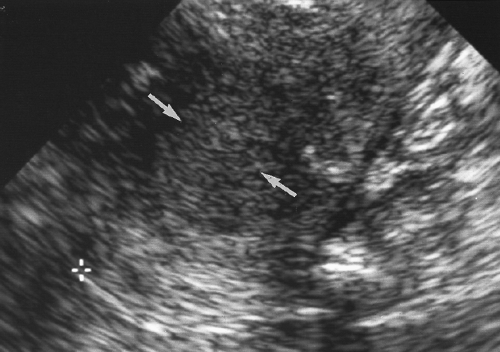
The ovaries are elliptical in shape and lobulated in contour. Follicles project from the outer cortex, and the stroma and blood vessels occupy the inner medulla (Fig. 5.6). The ovaries are attached to the uterus by a fold of the broad ligament called the ovarian ligament, and to the pelvic sidewall by the suspensory ligament of the ovary. The fallopian tubes in the broad ligament and the mesosalpinx drape over the ovary. The ovaries are
imaged in a shallow fossa anterior to the internal iliac vessels and medial to the external iliac vessels. The ovaries are displaced cephalad by increasing bladder distention. When the uterus is positioned toward the pelvic sidewall, the ipsilateral ovary is frequently located cephalad to the fundus. When the uterus is retroverted, the ovaries are usually ventral to the uterus at the level of the uterine body.
imaged in a shallow fossa anterior to the internal iliac vessels and medial to the external iliac vessels. The ovaries are displaced cephalad by increasing bladder distention. When the uterus is positioned toward the pelvic sidewall, the ipsilateral ovary is frequently located cephalad to the fundus. When the uterus is retroverted, the ovaries are usually ventral to the uterus at the level of the uterine body.
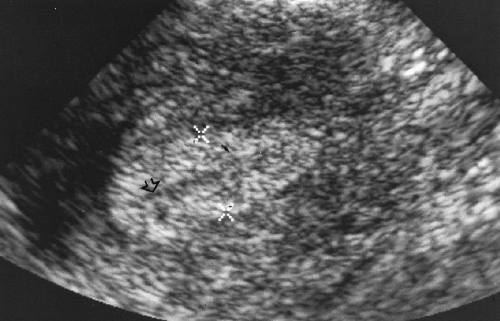
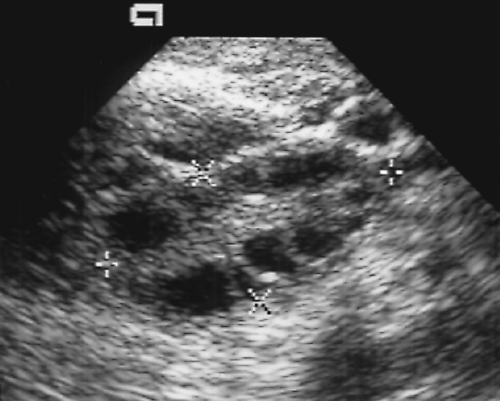 Figure 5.6 Normal Ovary. A normal ovary (marked by calipers) is shown in this transvaginal image. Normal follicles outline the periphery of the ovary, whereas echogenic stroma is seen centrally. |
The size and appearance of the ovaries change with age and with phase of the menstrual cycle (Table 5.3) [6]. In children younger than 8 years of age, the ovaries are homogeneous and solid [7]. Occasionally small follicles (<9 mm) are present. Normal follicles appear as anechoic, thin-walled cysts on the periphery of the ovary. In the follicular (proliferative) phase of the menstrual cycles, elevated levels of follicle-stimulating hormone and luteinizing hormone stimulate the development and enlargement of a variable number of follicles [6]. One follicle becomes dominant and matures as the Graafian follicle. This dominant follicle reaches a size of 20-25 mm, whereas most of the other follicles involute. Ovulation occurs when the dominant follicle ruptures, releasing the ovum and 15-25 ml of fluid into the peritoneal cavity. Mid-cycle pain, “mittelschmertz,” coincides with ovulation. The corpus luteum develops at the site of the ruptured dominant follicle. Blood clot and fibroblasts invade the collapsed follicle, which becomes intensely vascular and reforms a lymph and blood-filled cyst. The corpus luteum produces progesterone that supports the secretory endometrium to allow successful implantation if fertilization of the ovum occurs. The corpus luteum will degenerate in 14 days in the absence of an intervening pregnancy.
Size of the ovary is evaluated by measurement and calculation of volume using the standard formula (length × width × height × 0.52). Normal values are given in Table 5.3 [8, 9, 10]. The ovaries shrink with age after menopause [10].
Table 5.3: Normal Size of the Ovaries | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||
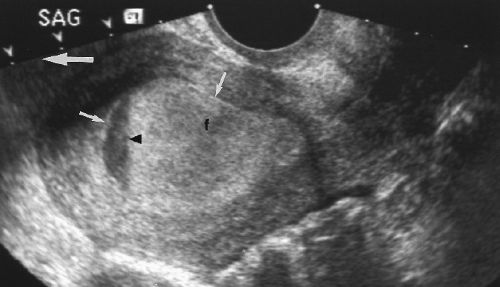
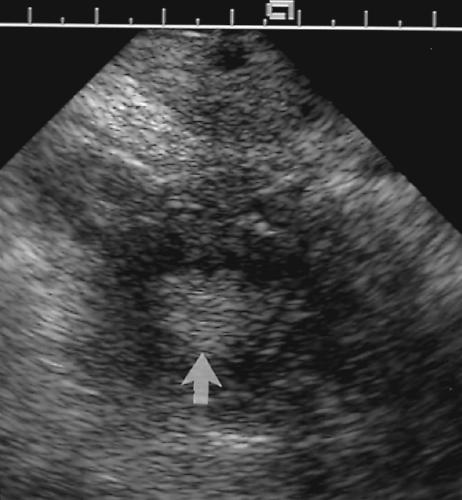
 Figure 5.7 Normal Fluid in the Cul-de-Sac. Anechoic fluid (f) is seen in the cul-de-sac posterior to the uterus (U). The fluid outlines loops of small bowel (b). |
The ovaries have a dual blood supply from the ovarian artery and from an adnexal branch of the uterine artery. Spectral Doppler shows high resistance flow in the first half of the menstrual cycle and low resistance flow in the second half after formation of the corpus luteum. The ovaries are hypovascular after menopause with flow detected only in the main ovarian artery.
The cul-de-sac frequently contains a small volume of fluid (~10 cc) that is best seen on TV US. A small volume of fluid is normal and physiologic (Fig. 5.7). The volume of fluid is increased with ovulation.
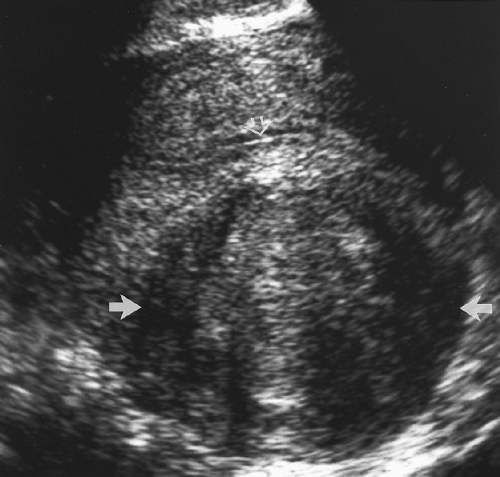
The normal vagina is a muscular tube in the midline of the pelvis extending from the vestibule of the external genitalia to the cervix. The cervix projects into the vaginal apex and is surrounded by vaginal recesses called the fornices. On sagittal images, the vagina appears as a tubular structure with hypoechoic muscular walls and a bright linear central echo caused by the apposing surfaces of the vaginal mucosa (Fig. 5.1). On transverse images (Fig. 5.8), the vagina appears as a flattened oval or flattened H-shaped structure with folds of vaginal mucosa laterally and the urethra coursing prominently in the anterior vaginal wall.
Uterus
Endometrial Abnormalities
Measuring Endometrial Thickness
Recurrence of vaginal bleeding in postmenopausal women is a common and potentially ominous complaint, with cancer as the cause of approximately 10% of cases. The majority of cases are caused by benign conditions including endometrial atrophy, hyperplasia, and polyps (Box 5.1). Conventional teaching has been that every postmenopausal woman with vaginal bleeding should undergo dilatation and curettage to sample the endometrium. US has provided a method of visualizing the endometrium and of being more selective in identifying patients for biopsy. US is used to assess the appearance and measure the thickness of the endometrium.
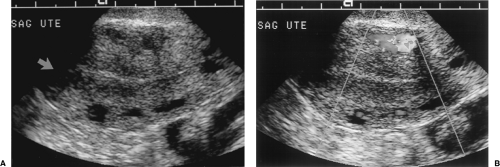
To properly use established criteria for basing the decision to biopsy on the thickness of the endometrium, the endometrium must be measured correctly. Endometrial thickness measurements are made “double layer” and include both the anterior and posterior endometrium (Fig. 5.9). The measurement is made perpendicular to the long axis of the uterine cavity where the endometrium is thickest and excludes any fluid in the cavity and the hypoechoic junctional myometrium. TV measurements are the most accurate. SHG aids in evaluation of the endometrium by outlining the endometrium surface and defining any polypoid protrusions [11]. A thin endometrium (<4 mm) or diffuse, smooth, regular thickening of the endometrium is the best predictor of benignancy [12]. Endometrial masses, irregular thickening, or focal thickening requires biopsy to exclude malignancy. In some patients, especially women with leiomyoma, the endometrium is distorted and is not adequately visualized for accurate measurement (~3% of patients) [13]. These women should undergo SHG or biopsy for diagnosis.
Criteria for biopsy as recommended by most authors is to biopsy patients with postmenopausal bleeding if the double-layer endometrial thickness exceeds 5 mm [13, 14]. In
patients who are asymptomatic, that is, specifically, without vaginal bleeding, the endometrium is considered normal up to 8 mm. Endometrial thickness >15 mm in a postmenopausal patient is very high risk for malignancy.
patients who are asymptomatic, that is, specifically, without vaginal bleeding, the endometrium is considered normal up to 8 mm. Endometrial thickness >15 mm in a postmenopausal patient is very high risk for malignancy.
Hormone Replacement Therapy
In postmenopausal women, hormone replacement therapy is commonly prescribed to abate menopausal symptoms and to prevent osteoporosis. The supplemental hormones have a small but notable effect on the appearance of the endometrium [15].
Unopposed estrogen therapy and concurrent estrogen and progesterone therapy increase endometrial thickness by 1.0-1.5 mm.
Sequential estrogen-progesterone therapy increases stripe thickness by 3.0 mm.
Endometrial Atrophy
Atrophy of the endometrium is the most common cause of postmenopausal bleeding (~60% of cases). The endometrium becomes inactive and atrophies as estrogen stimulation diminishes with menopause.
Cystic changes may occur and result in thickening of the endometrium.
Blood flow on Doppler US is minimal or absent.
Endometrial Hyperplasia
Endometrial hyperplasia is a proliferation of endometrial glands, which increase in size and assume an irregular shape. The hyperplasia is caused by unopposed stimulation of the endometrium by estrogen. Hormone replacement therapy with only estrogen is the most common cause of hyperplasia in postmenopausal women. In premenopausal women, causes include recurring anovulatory cycles, polycystic ovary disease, and obesity. Hyperplasia may be focal or diffuse, and is classified as adenomatous, cystic, or atypical adenomatous. Up to 25% of patients with endometrial hyperplasia with atypia will eventually develop endometrial cancer.
Diffuse or focal smooth thickening of the echogenic endometrium (Fig. 5.9).
Cystic changes are common.
Atypical hyperplasia often appears heterogeneous and irregular (Fig. 5.11).
Endometrial Polyps
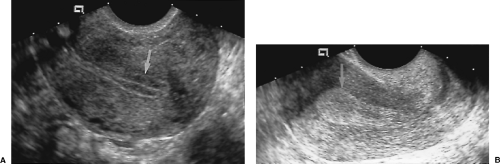
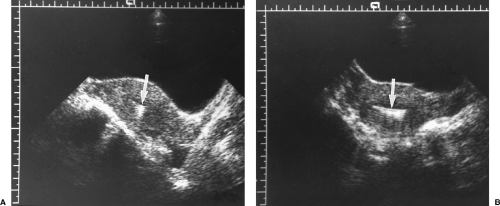
Endometrial polyps are localized, pedunculated, or broad-based growths of endometrial tissue that commonly cause bleeding. Polyps peak in prevalence in the fifth decade of life, but may be a cause of infertility in younger patients. Approximately 20% present after menopause. On routine TV sonography, the polyps appear as a non-specific echogenic thickening of the endometrium. However, when fluid is naturally present in the endometrial cavity or is introduced during SHG, an oval echogenic mass of intraluminal endometrium is evident. Treatment is resection by dilatation and curettage or by hysteroscopy.
Polyps may be pedunculated on a narrow stalk or broad-based (Fig. 5.13).
Vascular pedicle is demonstrable on color Doppler US.
Echogenicity is uniform, hyperechoic relative to myometrium and isoechoic to endometrium.
Cystic areas are occasionally present within the polyp.
Polyps are differentiated from submucosal leiomyomas by the uniform high echogenicity of the polyp (Fig. 5.13) [17].
Multiple polyps are present in 20% of cases.
Endometrial Carcinoma
Endometrial carcinoma is the most common gynecologic malignancy, occurring in 3% of American women. Most cases (80%) occur in postmenopausal women and most present with abnormal vaginal bleeding. Endometrial carcinoma is the cause of 7-30% of postmenopausal bleeding. US shows thickening of the endometrium that is often indistinguishable from hyperplasia and polyps. Signs that suggest cancer include inhomogeneous
echogenicity, irregular and poorly defined margins, and invasion of the myometrium [18].
echogenicity, irregular and poorly defined margins, and invasion of the myometrium [18].
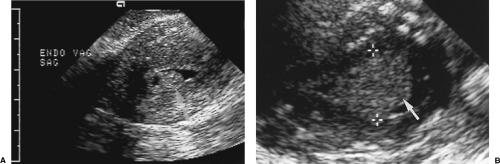 Figure 5.13 Endometrial Polyps. Two sonohysterograms (A, B) show pedunculated endometrial polyps (arrows) outlined by fluid instilled into the endometrial cavity. Note the high echogenicity of the polyp equal to that of the endometrium. Compare to the low echogenicity of the submucosal leiomyomas in Figure 5.16. Calipers (+) measure the size of the polyp in B. |
Thickened endometrium is a hallmark sign. Endometrial thickening >15 mm in postmenopausal women has a high risk of malignancy.
The endometrium is usually diffusely or partially echogenic (80-90%).
The endometrium is unevenly thickened and irregular in contour in 60-70% (Fig. 5.14).
Smooth, uniform, endometrial thickening indistinguishable from endometrial hyperplasia is present in 30-40%.
Poorly defined endometrium that is difficult to visualize suggests carcinoma (Fig. 5.15).
Cystic changes are present in 24%.
Some carcinomas are polypoid with a broad base.
Lack of distensibility of the endometrial canal may be evident on SHG [17].
Calcification is rare.
Tamoxifen-Related Endometrial Changes
Tamoxifen is an antiestrogen chemotherapeutic agent used in patients with breast cancer [19]. Although it is used for its antiestrogen, tumor-suppressive effects, the drug has proliferative effects on the endometrium and may promote endometrial tumor growth. Histologic changes reported with tamoxifen therapy include endometrial hyperplasia, endometrial polyps, and endometrial carcinoma [20, 21]. Cystic change is characteristic. Thickness of the endometrium increases with duration of tamoxifen therapy especially after 5 years.
The endometrium is irregularly thickened (usually >8 mm in postmenopausal patients).
Multicystic changes in the thickened endometrium is characteristic.
Endometrial polyps are common (33%).
Submucosal Leiomyoma
Leiomyomas located just beneath the endometrium are prone to ulceration and bleeding. They compress the endometrium, may bulge into the endometrial cavity, and may become a polypoid mass within the endometrial cavity. SHG is used to characterize the size and intraluminal extent of the mass so that surgical resection can be optimally planned using either a TA or hysteroscopic approach.
Hypoechoic mass just beneath the endometrium (Fig. 5.16).
The mass may cause acoustic shadowing.
The mass compresses the endometrium and may project into the endometrial cavity.
Vascularity, demonstrated by color Doppler, helps to differentiate leiomyomas from blood clots.
Low and heterogeneous echogenicity differentiates leiomyomas from echogenic endometrial polyps (Fig. 5.16) [17].
Leiomyomas commonly calcify.
Intrauterine Device
Intrauterine devices (IUDs) are currently in less common use because of complications of endometritis, ectopic pregnancy, and uterine perforation. US is effectively used to document IUD location when the IUD string is lost or to confirm proper IUD placement.
IUDs are seen as brightly echogenic structures in uterine cavity. The appearance depends upon the type of IUD.
The normal location for an IUD is centered in the uterine cavity near the fundus.
Copper-wrapped IUDs are intensely echogenic and cause comet tail artifacts (Fig. 5.17). Most are in the shape of a 7 or a T.
Lippes loops have a regular pattern of repeating echogenic foci that cast acoustic shadows.
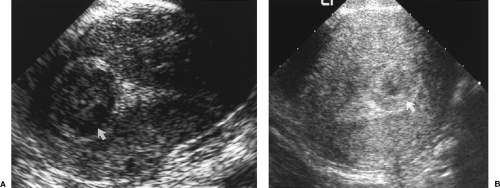 Figure 5.16 Submucosal Leiomyomas. Sonograms on two patients (A, B) show the characteristic low echogenicity of submucosal leiomyomas (arrows) that protrude into the uterine cavity. Compare with the appearance of endometrial polyps in Figure 5.12. |
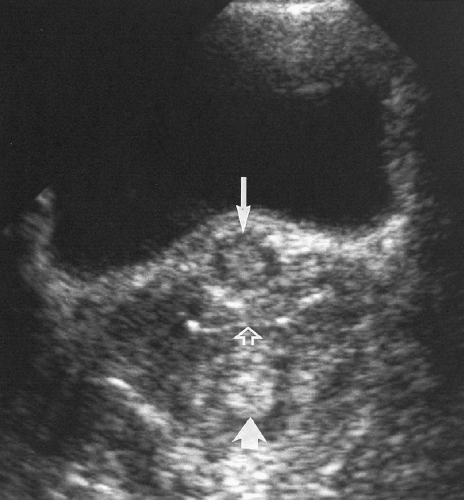
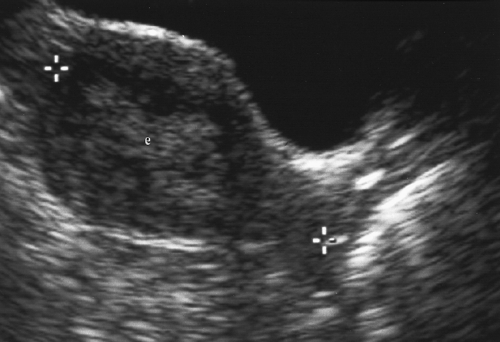
Fluid in the Endometrial Canal
A small volume of anechoic fluid in the endometrial canal is common in postmenopausal women (Fig. 5.4). It usually reflects cervical stenosis, or results from hormone replacement therapy [22]. According to one study, if the single layer endometrium measures 3 mm or less (<6 mm double layer), the endometrium is atrophic and inactive, so no further evaluation of the endometrium is necessary (Figs. 5.4, 5.10) [23]. Patients should be examined clinically for evidence of cervical carcinoma.
Intrauterine Adhesions
Adhesions are formed by scarring that fastens the walls of the uterus together and obliterates part or all of the uterine cavity [24]. Patients with a history of repeated uterine instrumentation or uterine infections are at highest risk. Patients present with amenorrhea or repeated spontaneous abortion.
Adhesions appear as an irregular hypoechoic bridge that interrupts the endometrium and is in continuity with the myometrium. Size, shape, and extent are variable.
Adhesions are best seen when fluid is present in the endometrial cavity either naturally or during SHG.
Endometritis
Endometritis occurs within the spectrum of pelvic inflammatory disease (PID). Puerperal endometritis is most common 2-5 days after delivery.
The endometrium is thickened and edematous.
Fluid is usually found in the endometrial cavity. It is commonly echogenic and layers producing fluid-fluid levels.
Gas in the endometrial cavity is found in 15% of patients. This is not a specific finding in the postpartum patient. In women with uncomplicated vaginal deliveries, gas may be found in the endometrial cavity in 19% of women during the first 3 postpartum days and in 7% of women for 3 weeks postpartum [25].
Myometrial Abnormalities
Leiomyomas
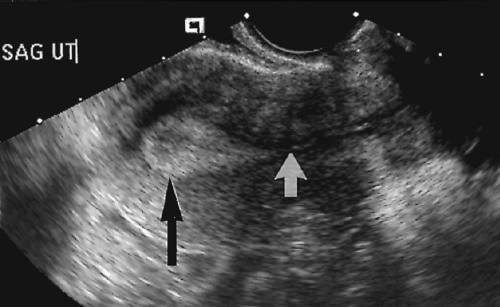
Leiomyomas are the most common tumor of the female pelvis, occurring in up to 40% of women older than age 35 [26] years. The tumors are composed of smooth muscle with variable amount of fibrous connective tissue. Most leiomyomas cause no symptoms, but they can cause menorrhagia, dysmenorrhea, irregular uterine bleeding, pelvic pain, infertility, and the discomfort of a large pelvic mass. Tumors grow in response to estrogen and typically enlarge during pregnancy and regress after menopause. Tumor location is described as intramural, submucosal (beneath the endometrium), or subserosal (on the surface of the uterus).
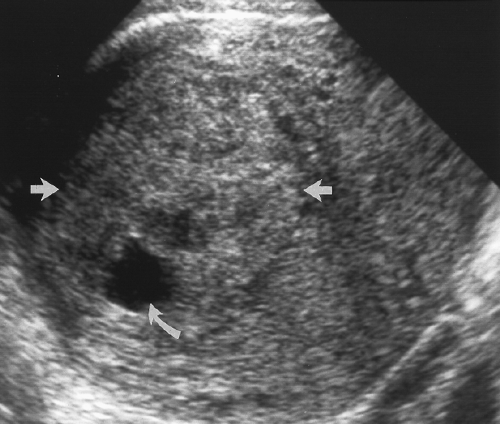 Figure 5.19 Cystic Change in Leiomyoma. An intramural leiomyoma (between arrows) with echogenicity slightly greater than normal myometrium shows an area of cystic necrosis (curved arrow). |
A round to oval, solid, hypoechoic mass is typical. Refractory acoustic shadowing produced by fibrous elements within the tumor is highly indicative of leiomyoma (Fig. 5.18) [27].
Inhomogeneous myometrium is produced by numerous small leiomyomas.
Tumors cause a focal bulge in the uterine contour (intramural leiomyoma), or compress and distort the endometrium (submucosal leiomyoma).
Necrosis, hemorrhage, and cystic changes are common (Fig. 5.19). Irregular, coarse calcifications are characteristic (Fig. 5.20).
Isoechoic tumors may be undetectable or cause only uterine enlargement.
Subserosal fibroids may become pedunculated and present as an adnexal mass. Diagnosis is made by color flow US demonstration of a vascular pedicle continuous with the myometrium (Fig. 5.21).
Lipomatous leiomyomas contain benign fat cells. With high fat content, the tumors are intensely echogenic.
Pedunculated serosal leiomyomas on a narrow stalk may parasitize blood supply from adjacent structures and detach from the uterus [28]. These are called parasitic leiomyomas.
 Figure 5.20 Calcification in Leiomyoma. A characteristic coarse calcification (arrow) is seen within an intramural leiomyoma that causes a focal bulge in the contour of the uterus. |
 Figure 5.21 Pedunculated Leiomyoma. A. Transvaginal US image shows a hypoechoic mass (outlined by cursors, x, +) adjacent to the uterus. B. Color Doppler image in the same location shows contiguous blood flow from the uterus into the mass, confirming a pedunculated leiomyoma (see Color Figure 5.21A, B).
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|